Western Flower Thrips in Greenhouses: A Review of its Biological Control and Other Methods
Prepared for the Web by Mark Hoddle, Extension Specialist and Director of Center for Invasive Species Research mark.hoddle@ucr.edu
Content Written by Roy Van Driesche, Professor of Entomology, University of Massachusetts, Amhearst vandries@cns.umass.edu
This fact sheet is intended to provide a detailed summary for growers and extension agents of our knowledge of western flower thrips, with emphasis on the potential for its biological control. Contributing authors include entomologists, plant pathologists, extension agents, and biological control specialists. We summarize our collective knowledge of the published literature and personal experience. This publication was funded with support from the New England Greenhouse Conference and the Massachusetts IPM Program.
Introduction |
|
Figure 1. Adult western flower thrips. |
Western flower thrips (WFT), Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) is a native insect of the western part of North America that was first reported in 1895. In the 1970s and early 1980s, this species spread throughout North America (Beshear 1983). Soon thereafter, it was found in Europe in Dutch greenhouses and has since become an exotic pest of greenhouse production in many countries throughout the world (Tommasini and Maini 1995). It is a damaging pest and virus vector on a variety of outdoor crops such as peanuts, tomatoes, lettuce, celery, peppers, peas, onions, apples and grapes (Robb 1989) and in greenhouse vegetable and flower crops, including, tomatoes, sweet pepper, cucumber, chrysanthemum, roses, impatiens, ivy geranium, petunia, gloxinia, orchids, dahlia, primula, gerbera, fuchsia, and African violet (Yudin et al. 1986; Parker et al. 1995; Daughtrey 1996; Daughtrey et al. 1995, 1997).
Pest Identification and Biology |
Recognition. Determination of thrips species requires good optics, a determination key, and the ability to recognize very small features on the body of the thrips. Parrella (1995) provides illustrations that separate WFT from other species. Knowing which species of thrips you have is important because some thrips do not vector the plant diseases that are the source of much of the damage caused by WFT.
To separate Frankliniella species from thrips in the genus Thrips (such as the onion thrips, Thrips tabaci Lindeman) there are three useful characters: number of setae ("hairs") on the pronotum (first body region behind the head), the number of pairs of setae between the compound eye and the simple eyes (ocelli) on the thrips head, and the presence of two complete rows of setae on the wings (Figs. 1 and 2). Although Mound and Kibby (1998) provide a relatively user-friendly, pictorially-based taxonomic key for identifying thrips, separating the various species of Frankliniella thrips is often complicated and requires slide-mounted specimens. See Parrella (1995) and Mound and Kibby (1998) for details.
Biology. WFT lays its eggs in plant tissue, using a blade-like ovipositor to insert eggs into leaves, buds, and petals. After egg hatch, there are two feeding life stages (called the first and second instar larvae), followed by two immobile non-feeding stages (the propupa and pupa) that both occur in the soil. Adults emerge from pupae and are winged. Adults feed, mate, disperse, and females lay eggs. Laboratory methods to mass rear and synchronize the developmental stages of WFT have been developed (see Loomans and Murai 1997 for a review).
Although a complex of three color types of WFT occurs in the western United States (its native area) (Bailey and Smith 1956), only one of these types has spread internationally (with the exception of one population in New Zealand) (de Kogel et al. 1997).
Times need for development of WFT life stages have been measured on several crop species, including cucumber (Gaum et al. 1994, van Rijn et al. 1995), French bean (Gerin et al. 1994) and chrysanthemum (Katayama 1997). Exact developmental times will depend both on temperature and the host plant on which the thrips are feeding. At low temperatures (59°F [15°C]), WFT requires 13 days or more to complete a generation (the exact value depending on how "generation" is defined) and the rate of population increase from one generation to the next is low (only 1.02, where a value of 1.0 indicates no population growth, 2 indicates a doubling in one generation, etc.). However, at 86°F (30°C), the generation time decreases to 4.3 days and the population increases 8.5 fold with each generation.
Once mated, WFT produce offspring biased toward females, ranging from 58-70% female depending on age of the mother, the local thrips population density, and perhaps temperature (Higgins and Myers 1992, Gaum et al. 1994, Katayama 1997).
The developmental rates, fecundity and longevity of WFT are affected by many factors, including temperature, day length, and the plant species it is feeding on (Soria and Mollema 1992, Gaum et al. 1994, Brødsgaard 1994a, Katayama 1997). The presence of pollen (a food source for WFT) especially affects these factors (Trichilo and Leigh 1988). Compared to onion thrips (another important pest thrips in some greenhouse crops), WFT develops more rapidly, but lays fewer eggs, a higher proportion of which are males. These latter two factors outweigh WFT’s faster development, causing its rate of population growth to be slightly lower than that of onion thrips (van Rijn et al. 1995).
Damage and Relation to Plant Diseases |
Direct damage. Thrips feed by using their mouthparts to pierce plant cells and suck out their contents. Damaged plant cells collapse, resulting in deformed plant growth, flower deformation, or silvered patches and flecking on expanded leaves. If thrips feed on the surface of expanded leaves or petals, damage appears as small scars or silvered patches. Greenish-black fecal specks are left by the thrips on plant surfaces as thrips feed. If thrips feed within developing buds, the damaged cells fail to grow as the leaf or flower expands, resulting in deformed leaves or flowers. Flower buds may abort in heavy infestations. Some plants develop a local dead or discolored spot where thrips eggs have been inserted into plant tissue.
In chrysanthemum and gerbera flowers, feeding results in distorted petals, discoloration, and extensive streaking. In geranium, feeding causes young leaves to grow in a deformed manner, curling upwards, and to have whitish bumps on the upper leaf surface (Tommasini and Maini 1995). In young poinsettia and impatiens, thrips feeding distorts the outline of developing leaves. In African violets, thrips feeding ruptures pollen sacs, spreading loose pollen over flowers.
Vectoring of plant diseases. Western flower thrips is a vector of many plant diseases, the most important of which for greenhouse producers are two plant viruses in the genus Tospovirus: impatiens necrotic spot virus (INSV) and tomato spotted wilt virus (TSWV) (German et al. 1992, Ullman et al. 1997). Prior to 1989, only one WFT-vectored tospovirus (TSWV) was known. In 1989, a new serotype was discovered in impatiens that became known as TSWV-I (for impatiens), in contrast to the common strain first characterized from lettuce that was known as TSWV-L (for lettuce). In 1990, TSWV-I became formalized as INSV (Law and Moyer 1990). Studies before 1990 must be carefully interpreted to identify which virus is being discussed. In North American greenhouse plants, INSV is the predominant of the two viruses, but in Europe, TSWV is the common form. This is likely related to the closer association of TSWV with vegetables and INSV with ornamentals (Daughtrey et al. 1997). (However, on two ornamental crops, dahlias and chrysanthemums, TSWV is the more important virus).
These tospoviruses are acquired only during the larval stages by WFT that feed on diseased plants. The viruses then multiply inside the salivary glands and other tissues of the thrips (Wijkamp et al. 1993, van de Wetering et al. 1996a) and are later transmitted to new plants by the feeding of the infected thrips after it has reached the adult stage. Male WFT transmit TSWV much more efficiently than females (van de Wetering et al. 1996b). A minimum of 15-30 minutes of feeding is required for transmission to healthy plants (Sakimura 1962ab). Transmission readily occurs in commercial greenhouse crops of ornamentals and tomatoes (Allen and Broadbent 1986), causing important economic losses (Daughtrey et al. 1997).
Symptoms of these diseases vary widely among plant hosts. Daughtrey et al. (1997) provide a table comparing symptoms of INSV and TSWV for tobacco, tomato, datura, gloxinia, chrysanthemum, petunia, and impatiens, as well as color photographs of symptoms on some plants. Many flowering potted plants can be infected by these viruses, including Anemone, Aquilegia, Begonia, Browallia, Calceolaria, Campanula, Capsicum, Chrysanthemum, Clerodendrum, Eustoma, Fuchsia, Gardenia, Gerbera, Hosta, Impatiens, Kalanchoe, Lantana, Lycopersicon, Mimulus, New Guinea impatiens, Petunia, Ranunculus, Saintpaulia, Schlumbergera, Sinningia, Solanum, Schizanthus, and Streptocarpus (Daughtrey et al. 1995, 1997).
Monitoring and Controlling Western Flower Thrips |
Monitoring. WFT population monitoring is necessary to detect incipient WFT problems in crops and to determine if control actions have been effective (see Chs. 16 and 18 in Lewis, 1997 for examples of thrips monitoring programs). Early detection is important because symptoms of feeding often go unnoticed until serious damage has occurred. Also, small populations are easier to control than large ones.
Counting thrips on plants, however, is time-consuming and is not cost-effective in commercial crops (Shipp and Zariffa 1991). Instead, thrips counts on plants can be based on a presence-absence assessment, in which the sampler takes note of the proportion of samples with thrips, rather than actually counting numbers of thrips in each sample (Schmidt and Frey 1995).
Alternatively, thrips may be monitored with sticky traps or "the tapping method." Tapping the flowers or foliage of a few plants gently over a sheet of white paper will dislodge thrips and make them visible. Such plant tapping can be used to determine if thrips are present, and to gain a rough estimate of their numbers.
For more precision, sticky traps may be used. (Some scouts like to use both sticky traps and the tapping method. It is not unusual for tapping of plants suspected of being infested to detect thrips before any show up on randomly placed sticky trap cards.) Traps should be placed just above the crop canopy, about one per 200 square meters (approx. 2,000 square feet) in large houses. In houses under 2,000 square feet, use a minimum of three traps, regardless of house size. Place traps near doors, vents, and over thrips-sensitive plants. Both yellow and blue sticky traps will catch WFT. Blue traps are somewhat more efficient; however, yellow traps are also attractive to whiteflies and other flying greenhouse pests, which may be an important feature for overall pest monitoring.
Counts on traps are meaningful in two ways. First, trends of trap captures (after trap counts are graphed against date of capture) tell growers in what direction the population is changing and at what speed. Trend information is especially useful in determining the efficacy of a control measure that has been applied (by comparing counts before and after the treatment). Whether or not a control measure is needed, however, cannot be told only from trend information. Rather, a damage threshold must be known, i.e., the number of thrips per trap that indicate that damage is likely to occur unless the population is reduced.
Some thrips damage thresholds have been published. For example, thresholds for thrips captures on the blue trap Rebell blu® have been developed in Switzerland for nine ornamental species. These thresholds range from 10 thrips per trap per week to over 40, depending on the sensitivity of the crop (Schmidt and Frey 1995). Shipp et al. (1998) have defined economic injury levels for WFT on sweet pepper in Canadian greenhouses.
However, thresholds are very sensitive to the particular conditions at hand, including the crop, variety, local market standards, and, very importantly, whether or not INSV or TSWV is present at the site. Rather than rely on threshold values developed at some other site, under conditions that are likely to differ from one’s own, growers may do better by using thrips counts from their own monitoring efforts in past crops. If thrips counts and information on crop quality and losses (to thrips damage or Tospoviruses) are kept, this information will help each grower identify what thrips level is sufficient to cause losses, given the grower’s own conditions and desired level of plant quality for the local market.
Growers who use biological control on long-term crops may want to use a threshold that takes into account the level of natural enemies present as well as the pest levels. This may take the form of a ratio of the number of natural enemies to the number of pests. For thrips, this might be the ratio of predacious bugs or mites per leaf to the number of thrips per leaf.
The time spent counting insects on sticky traps can be reduced, with yellow traps at least, by counting only the insects on a one inch wide (2.3 cm) strip, rather than the whole trap surface (Heinz et al. 1992). Thresholds would, of course, have to be changed proportionately.
Effects of various potential trap shapes, sizes and background colors have been studied (Vernon and Gillespie 1995). Trap catches of WFT increase in direct proportion to trap area, so actual trap size is not important in increasing trap efficiency. Catches of cylindrical and flat traps, of the same area, do not differ. Traps placed against backgrounds of sharply contrasting color catch more thrips than traps against backgrounds of the same color.
Flower odors are highly attractive to WFT, so that, in blooming crops, trap catches decline because of competition between real flowers and traps. Addition of floral odors to traps, so far, however, has been ineffective in increasing trap attractiveness under these circumstances (Frey et al. 1994). (Combining the use of flower or plant tapings with trap counts may overcome some of the limitations present in the use of traps alone).
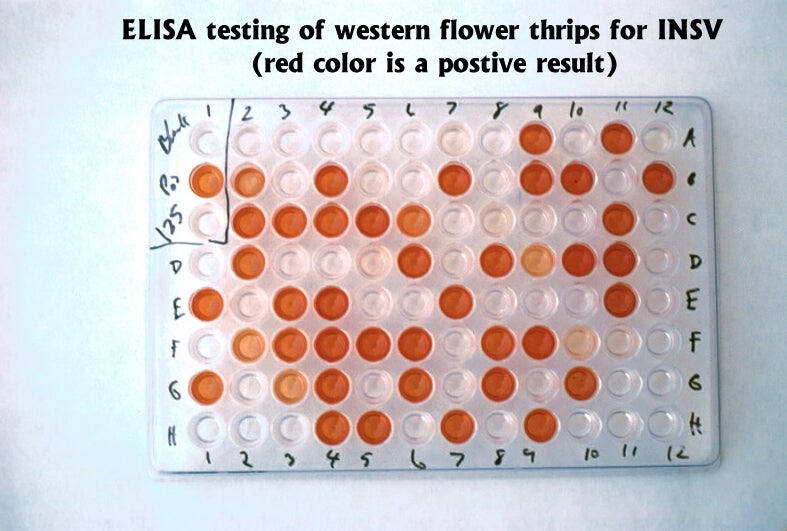
In addition to knowing the number of thrips present in a crop, it is also important to know if thrips are infected with tospoviruses. Methods have been developed that use ELISA (enzyme-linked immunosorbent assay) and dot blot tests to determine if individual thrips are virus-infected or not (Cho et al 1988, Rice et al. 1990). Commercial test kits for grower use are available. Virus remains detectable in dead thrips on sticky cards for at least two weeks.
Tospoviruses can also be detected in greenhouse crops by using indicator plants such as fava bean (any dwarf variety) or petunia (Allen and Matteoni 1991). Petunia (Petunia X hybrida Hort. Viulm.-Andr.) varieties such as "Summer Madness", "Super Magic Coral", or "Red Cloud" are good choices. Place a yellow or blue non-sticky card on a stake in the plot to enhance the indicator plant’s attractiveness to WFT. Check indicator plants for the characteristic dark-ringed lesions that develop around thrips feeding scars if toposviruses are present. If petunias are used, keep flowers removed because the thrips will preferentially feed on flowers, but these do not show virus symptoms. INSV does not become systemic in these petunia cultivars; therefore, infected plants that might result from their use as indicator plants will not become a virus reservoir within the greenhouse.
Prevention. It is usually easier to prevent an infestation than to manage an established one. Growers should try to reduce the number of thrips present in greenhouses at the end of the growing season. Otherwise, thrips will overwinter in the greenhouse and this may result in a large population at the beginning of the next growing season. Growers should also try to avoid buying thrips-infested plants and introducing them into their greenhouses. Tapping flowers of incoming plants over a white piece of paper is a fast method to screen incoming shipments. If present, thrips will be dislodged and are visible on white paper.
WFT prefer to feed on flowers, so the longer a crop can be grown without flowering, the lower the thrips populations will be. For example, stock plants of some vegetatively-propagated floral crops can be grown flower-free. In such cases, suppressing flowering, or hand picking and disposing of flowers as they develop, can make control of WFT populations easier.
Weeds such as Galinsoga sp. and chickweed (Stellaria media) can serve as important reservoirs of both tospoviruses and thrips in greenhouse crops (Stobbs et al. 1992). Weeds inside and outside (within a 10 meter perimeter) of a greenhouse should be destroyed as an important part of virus and thrips suppression (Cho et al. 1986). Flowering ornamental plantings around greenhouses should also be removed to reduce the chances for development of large populations of thrips adjacent to greenhouses.
Mechanical exclusion of thrips can be achieved with appropriate screening. Screening with 135 micron size openings can substantially reduce thrips entry to greenhouses (Bethke et al. 1994, Baker et al. 1995). However, when virus-susceptible propagation material is being produced, 100% exclusion is needed, requiring smaller size openings in screening, or use of solid materials.
Exclusion of WFT is not, however, simply a matter of placing a fine screen over vents and air intakes. Important points to be considered in the design of an exclusion screening system include:
- Screening will only be helpful if the source of an infestation is from thrips migrating in from the outside and not from infected plant material within the greenhouse.
- Screening of all openings is usually necessary (i.e., doors, vents).
- Greenhouses cooled by passive ventilation systems are difficult to screen without making structural changes to compensate for reductions in air flow associated with screening.
- Guidelines of the National Greenhouse Manufacturers Assoc. should be consulted when installing insect screens in mechanically vented greenhouses (Robb and Parrella 1995).
Plant resistance. Genetic resistance to damage from WFT feeding has been sought in tomatoes (Kumar et al. 1995), cucumber (Soria and Mollema 1992), sweet pepper (Fery and Schalk 1991), and chrysanthemum (de Jager and Butot 1992, de Jager et al. 1995). While potentially a useful factor in IPM programs to manage WFT, use of resistant cultivars has not resolved the problem, and variation between cultivars in their other qualities are important influences on production decisions. In addition, stable resistance to TSWV has been difficult to obtain (Best 1968).
Chemical control. Chemical control is the most frequently used method to suppress WFT in greenhouses (Oetting 1988, Helyer and Brobyn 1992). Successful use of insecticides for WFT control requires attention to the issues of pesticide choice, coverage, phytotoxicity, and resistance.
When insecticides are applied as foliar sprays, it is important to use equipment producing small droplets (<100 microns) in order to secure good coverage and better penetration into plant parts where most thrips feed. Insecticide applications should be repeated on a five day schedule for at least three applications. Addition of a pyrethrin-like insecticide (e.g., resmethrin and others) to the application may increase contact of thrips with residues by inducing movement in thrips.
Because most WFT pupate in soil or potting media, an alternative to foliage application is to apply pesticides to soil (Helyer et al. 1995). However, at any one time only a small percentage of the total WFT population in a greenhouse crop will be in the soil as pupae. Therefore even highly effective measures taken against this part of the population, unless repeated frequently, will fail to control the population. Also, few pesticides are currently labeled for use in soil in greenhouses, further restricting this option.
Because of the diverse range of ornamental plants produced in greenhouses, application of pesticides often poses some risk of phytotoxicity. This can be the case in chemical control programs directed against WFT. For example, on gloxinia (Sinningia speciosa), unacceptable levels of phytotoxicity to flowers (over 4%) were reached when chlorpyrifos was used at 10-day intervals or when abamectin or cyfluthrin were used at 5-day intervals (Nasruddin and Smitley 1991).
Pesticide resistance. A further limitation to the effective suppression of WFT with chemical pesticides is the development of resistance (Robb et al. 1995). Immaraju et al. (1992) found high levels of resistance in certain WFT populations to permethrin, bifenthrin and abamectin, and moderate-to-high resistance to methomyl. Resistance was relatively low to chlorpyrifos. Brødsgaard (1994b) found that five populations of WFT from European and African greenhouses were resistant to endosulfan, methiocarb, and acephate, relative to a field population of WFT. Resistance was also stable and one resistant strain remained resistant in a pesticide-free environment for 4 years (approximately 100 generations). Zhao et al. (1995) found that WFT from five commercial greenhouses in North America were resistant to diazinon, methomyl, bendiocarb, and cypermethrin.
To delay development of resistance, a standard recommendation is to use long-term rotations. Use a given effective insecticide for several generations of WFT (3-4 weeks), then rotate to another insecticide with a different mode of action (different chemical class) for several WFT generations. Then, rotate to a third class of insecticides, and finally, return to the original material and repeat the whole process.
While resistance is of concern, in greenhouse crop production, failure of chemical control is, however, more likely to be due to poor timing or poor coverage, and these factors should be considered before assuming that a resistant thrips population has developed.
Biological Control Approaches |
Natural enemies that have been examined for their abilities to suppress WFT populations in greenhouse crops include predacious bugs, predacious mites, parasitic wasps, pathogenic fungi, and nematodes. The later two groups are dealt with in a separate section on pathogens.
Outdoor crops and habitats have been surveyed in Australia (Goodwin and Steiner 1996), Mediterranean Europe (Loomans 1991, Riudavets and Castañé 1998) and California (Heinz et al. 1996, Loomans and van Lenteren 1996) to identify potential new natural enemies that might be used for augmentative biological control against WFT in greenhouse crops. Past published records of WFT predators (Riudavets 1995, Sabelis and van Rijn 1997) and parasitoids (Loomans and van Lenteren 1995; Loomans et al. 1997) have been reviewed.
Predacious bugs. The principal predacious insects associated with WFT populations have been anthocorid bugs (minute pirate bugs, Orius spp.) and mirids (plant bugs, including the species Dicyphus tamaninii Wagner and Macrolophus caliginosus Wagner (Riudavets and Castañé 1998). Several species of Orius have been recorded as predators of WFT and some have been used in greenhouse trials to assess their efficacy, including Orius laevigatus (Fiever), O. majusculus (Reuter), O. armatus, O. heterorioides, O. tantillus, and O. insidiosus (Say) (Ferguson and Schmidt 1996, Goodwin and Steiner 1996, Riudavets and Castañé 1998). In surveys in Spain, four bugs, O. laevigatus, O. majusculus, D. tamaninii, and M. caliginosus, accounted for 95% of the WFT predators encountered. All four of these species were able to complete their development on a diet of WFT larvae. Adults ate more WFT larvae than did immature bugs. The anthocorids (Orius spp.) laid more eggs than did the mirids (D. tamaninii, and M. caliginosus) (Riudavets and Castañé 1998).
Orius bugs have received more careful study than other Hemiptera. Studies have included efforts to locate non-diapausing strains that would continue to actively reproduce in winter under short day length conditions in greenhouses (Tommasini and Nicoli 1996). Efforts have also been made to develop optimal conditions for mass production of Orius species (Blümel 1996), including the possible use of artificial substrates for oviposition (Castañé and Zalom 1994). The relative suitabilities of cucumbers, tomatoes, and sweet peppers for foraging of O. insidiosus have been compared and cucumber found to be somewhat less suitable (Ferguson and Schmidt 1996). The number of prey eaten by O. insidiosus when offered various numbers of WFT has also be quantified (Coll and Ridgeway 1995).
Predacious mites. Many species of phytoseiid mites have been recorded as eating some stages of WFT (Sabelis and van Rijn 1997). Kostiainen and Hoy (1996), in a bibliography compiling all the literature from 1960 through 1994, list the following species as predators of WFT: (1) Euseius stipulatus, (2) Metaseiulus occidentalis (Nesbitt), (3) Amblyseius andersoni (Chant), (4) Amblyseius barkeri (Hughes) (A. mckenziei), (5) Amblyseius californicus McGregor, (6) Amblyseius (Neoseiulus) cucumeris (Oudemans), (7) Amblyseius (Iphiseius) degenerans Berlese, (8) Amblyseius (Euseius) hibisci (Chant), (9) Amblyseius limonicus s.s. Garmon and McGregor, (10) Amblyseius scutalis (Athias-Henriot), and (11) Amblyseius (Euseius) tularensis (Congdon). The greatest number of studies have concerned A. barkeri (19 articles) and A. cucumeris (49 articles). Riudavets (1995) provides detailed information from the literature on these two important species. The taxonomists who study phytoseiid mites currently do not agree as to the generic placement of species and the same mite may appear in different articles with different generic names (e.g., Amblyseius degenerans and Iphiseius degenerans). Also, several different mites may in some cases bear the same name if "cryptic species" have not been recognized and thus not separately named.
A general caveat about predacious mites is that many, if not most, eat a variety of materials, including plant pollen and fungal spores, in addition to arthropods. Consequently, the crop plant on which mites are expected to provide biological control of a pest can strongly influence their success or failure. Biological control of WFT, for example, works better on sweet pepper than on many other crops because populations of predatory mites build up by feeding on the abundant pollen supply on this plant. In contrast, on plants which are not producing pollen, or on short cycle crops in which time for population increase is lacking, mites may fail to provide biological control of pests. These general limitations should be kept in mind for all the mite species discussed below.
Amblyseius cucumeris. When feeding on WFT, A. cucumeris completes its life cycle in 11.1, 8.7, and 6.3 days at 68, 77, or 86°F (20, 25 and 30°C). The lower threshold for larval development is 46°F (7.7°C). A pair of mites on average eats 5 first instar larvae of WFT each day, but older life stages are immune to attack because of their larger size and more effective defensive behaviors. Amblyseius cucumeris cannot survive below 32°F (0°C), but can be stored for up to 10 weeks at 48°F (9°C) with only 37% mortality. The critical photoperiod for diapause induction is 12.5 hours day light, given a day temperature of 72°F (22°C) and night temperature of 63°F (17°C). This predator aggregates on high density patches of WFT larvae, where it feeds on young larvae and lays its eggs (for above details, see references cited in Riudavets 1995, and Sabelis and van Rijn 1997).
Amblyseius barkeri. This is a widely distributed species that has been found on many greenhouse crops, preying on WFT and other thrips species. The distribution of this mite on plants is similar to that of WFT and the mite lays its eggs on the undersides of leaves near the top of the plant. An average of 2 eggs are laid per female per day. At 77°F (25°C), 6.2 days are required to complete the life cycle. Female mites live 30 days and over this time consume about 89 first instar thrips larvae. Older larvae and adults are not subject to attack. There is some doubt whether this mite can successfully complete its life cycle solely on a diet of WFT (for above details, see references cited in Riudavets 1995, and Sabelis and van Rijn 1997).
Other Amblyseius species: A. degenerans, A. hibisci, and A. limonicus. Because control achieved by A. cucumeris or A. barkeri has not been adequate on some crops, the biologies of other species of predacious mites have been studied, seeking a superior WFT predator. Critical attributes are those that determine the ability of the species to survive under greenhouse conditions, to increase to high densities, and to consume the greatest number of thrips. Van Houten et al. (1995a), seeking to find a more effective predator than A. cucumeris, studied five subtropical species: A. hibisci, A. degenerans, A. scutalis, A. tularensis, and A. limonicus.
Results of van Houten's tests showed that A. limonicus had the highest predation and oviposition rates and did not go into diapause under short winter day lengths. However, its eggs were relatively sensitive to low humidities. Amblyseius degenerans and A. hibisci eggs were less sensitive to low humidities and these mites exhibited rates of predation and oviposition that were intermediate between A. cucumeris and A. limonicus. Amblyseius hibisci and A. degenerans were considered by the authors to be good candidates for WFT control under conditions of short day length and low humidities. In a subsequent comparison of A. cucumeris and A. limonicus in a cucumber greenhouse in Holland, A. limonicus was able to suppress WFT populations, whereas A. cucumeris was not (van Houten 1996).
Presently, only one thrips parasitoid is sold commercially: Thripobius semiluteus Boucek (Hunter 1997). However, this species attacks only the greenhouse thrips (Heliothrips haemorrhoidalis [Bouché]) and does not attack the more important western flower thrips (Loomans and van Lenteren 1995). Basic research to assess other species of thrips parasitoids as potential biological control agents for WFT is needed and some is underway. Loomans and van Lenteren (1995) provide a detailed summary of knowledge of thrips parasitoids.
Thrips parasitoids are found in three families (Eulophidae, Trichogrammatidae, Mymaridae) and several genera. Eulophid wasps in the genera Ceranisus, Thripobius, Goetheana, Entedonastichus, and Pediobius are solitary internal parasitoids of thrips larvae. Of these genera, only Ceranisus and Goetheana contain species known to attack thrips related to WFT at the genus or subfamily level. Trichogrammatid and mymarid wasps in some genera are recorded as parasitoids of thrips eggs. Some species of the trichogrammatid genus Megaphragma attack thrips in the same genus as WFT.
Of these various parasitoids, as potential biological control agents for WFT, most attention has been directed toward eulophids in the genus Ceranisus, principally C. menes (Walker) and C. americensis (Girault) (Loomans et al. 1995a, Loomans and van Lenteren 1996, Castineiras et al. 1996). These parasitoids lay their eggs in young thrips larvae. Additional larvae are also killed by "host feeding" (ovipositor insertion without egg laying, followed by wasp feeding on host fluids from wounds, followed by host death). Developmental times of the immature stages of Ceranisus species are relatively long, 25-50 days depending on the host species, if temperatures are in the 20-25°C range, and much longer (up to 130 days) at temperatures below 20°C. The long developmental time of these parasitoids, relative to that of WFT itself, limits their effectiveness as biological control agents. Further investigations of new species or races of thrips parasitoids, perhaps subtropical or tropical species, might lead to discovery of wasps with faster developmental rates that might be more effective.
Efficacy Trials With Predators and Parasites
Predacious bugs. Several species of Orius bugs have been tested for control of WFT on sweet pepper and cucumber, including O. tristicolor (Gilkeson et al. 1990, Steiner and Tellier 1990), O. insidiosus (van den Meiracker and Ramakers 1991), and O. laevigatus (Chambers et al. 1993) and on strawberry (Frescata and Mexia 1996). Chambers et al. (1993) in testing O. laevigatus in cucumber and pepper in the United Kingdom found it was not possible to establish the predator on a cucumber crop, but breeding populations established satisfactorily in the flowers of sweet peppers (Capsicum). On peppers, releases totaling 1-2 predators per plant resulted in good thrips control over several months, providing initial thrips numbers were low. Early season supplementary lighting using tungsten bulbs to extend the photoperiod ensured good control of thrips on peppers in February and March by preventing diapause and thus promoting breeding by O. laevigatus on the crop. In Israel, Rubin et al. (1996) found that O. laevigatus failed to control WFT in pepper during the winter, but that better results appeared to occur with O. albidipennis (Reuter).
Many factors contribute to variation in efficacy (for example, the availability of alternative foods such as pollen), but the tendency of a given species or population of Orius to enter diapause (a state of physiological arrestment with little feeding or egg laying) appears to be a particularly important issue (van den Meiracker 1994). Careful studies of the minimum day length tolerated before induction of diapause have found great variation among Orius species. Specifically, van den Meriacker (1994) found that for O. insidiosus the critical photoperiod was between 11 and 12 hours, and for O. majusculus it was between 14 and 16 hours. In O. tristicolor, over 50% entered diapause for all day lengths below 16 hours (van den Meiracker 1994). In marked contrast, O. albidipennis had little tendency to enter diapause (fewer than 25%) at most day lengths, down to 8 hours. Different populations of the same species of Orius collected in geographic regions differing in latitude can also be expected to differ in their critical day length diapause responses. Tommasini and Nicoli (1996) found that egg laying by O. laevigatus from southern Italy (37o n. l.) was reduced less by short day lengths than was egg laying of a population from northern Italy (44o n. l.).
Less work has been done to explore the ability of Orius species to suppress WFT damage in floral crops, compared to the previously discussed vegetable and fruit crops. Fransen and Tolsma (1992) found that release of one O. insidious per chrysanthemum plant every other week reduced thrips damage from 40-90% in untreated controls to 5-20%. Smitley (1992) found that O. insidiosus was unable to reduce WFT in marigolds below 3.5 thrips per flower, a density that greatly surpasses acceptable levels for this bedding plant. While providing some reduction, such results have suggested that Orius species tested so far on ornamentals provide too little control to be acceptable and are uneconomical for use (Parrella and Murphy 1996). Further trials, especially of non-diapausing species such as O. albidipennis, might be warranted. It may also be of value to test releases of Orius spp. in combination with other natural enemies.
The effects of 31 pesticides on nymphal survival of O. laevigatus and on the oviposition of adults developing from surviving nymphs have been determined (van de Veire et al. 1996). Most fungicides had no toxic effects. Miticides had variable toxicity, and some insect growth regulators, such as azadirachtin, were not harmful.
Predacious mites. Biological control of thrips with predatory mite releases began in European sweet pepper crops infested with the onion thrips, Thrips tabaci. Following several years of research, commercial use of Amblyseius cucumeris and A. barkeri (mckenziei) was begun in 1985 in Holland with releases on about 25% of the Dutch pepper acreage under glass (de Klerk and Ramakers 1986). Amblyseius cucumeris releases expanded to about 60% of the Dutch pepper crop within one year, and control was judged satisfactory in about 80% of cases. At that time, however, control in cucumber was insufficient and this predator could not be recommended for commercial use in cucumber (Ravensberg and Altena 1987).
Two problems with use of predatory mites for thrips control in European vegetable glasshouses were recognized: mite diapause in winter and lack of pollen resources in some crops. In addition, invasion by WFT (and its replacement of onion thrips as the pest of greatest concern) redirected the need for control to this new thrips species, whose biology differs from that of onion thrips.
Use of initial strains of A. cucumeris during fall and winter in European and North American glasshouses failed to control WFT because short daylengths (below 11 hours light), together with low night temperatures, induced diapause in adult female mites, causing them to cease oviposition. Mite populations then ceased expanding and were quickly ineffective against thrips populations that continued to expand (Morewood and Gilkeson 1991, Rodriguez-Reina et al. 1994). To overcome this problem, populations of A. cucumeris from various countries were examined, and a non-diapausing strain (from New Zealand) was located (van Houten et al. 1995b) and found to be more effective on pepper than the earlier diapausing strain (van Houten and van Stratum 1995).
Use of non-diapausing strains of A. cucumeris, while relatively successful on sweet pepper, has continued to be less successful on cucumber (van Houten 1996), presumably because cucumber plants provide less pollen for mites (Ramakers 1995).
Improved control of thrips on cucumber was achieved through use of other species of Amblyseius mites. A non-diapausing strain of the subtropical species A. limonicus was identified in laboratory tests as having the highest predation and oviposition rates of five candidate species (van Houten et al. 1995a). Concerns that it would not perform well under low humidities proved not to be important in Dutch cucumber houses. Tests have since demonstrated that A. limonicus is able to suppress WFT on cucumber, under conditions where A. cucumeris failed to do so (van Houten 1996).
Another predator species, A. degenerans, also has been found to be highly effective against WFT, but high rearing costs have prevented release of this species in adequate numbers. However, a cheap rearing method based on use of flowering castor beans (Ricinus communis) has been developed (Ramakers and Voet 1995). Also, an in-greenhouse rearing process (called the "banker plant system") has been developed (Ramakers and Voet 1996). This process involves placing castor bean plants bearing thriving colonies of A. degenerans into the greenhouse at the beginning of the crop. Over time, mites move off banker plants onto the crop, a process that can be accelerated by increasing the number of banker plants and moving banker plants to new locations every few weeks.
As with most comparisons between vegetable and flower crops, the lower economic threshold for injury on flowers makes the achievement of adequate pest suppression by biological control in ornamentals more difficult. However, Gill (1994) found that releases of A. cucumeris in crops of ornamental bedding plants (via the sachet release system) reduced the number of pesticide applications needed for WFT control from 5 to 0.4. In chrysanthemum, Hessein and Parrella (1990) found that releases of A. cucumeris and A. barkeri were not able to suppress WFT below a level of 2-7 per leaf, a number too high for this crop. The potential levels of effectiveness of A. limonicus and A. degenerans need further investigation.
Representatives of Koppert Biological Systems have expressed the opinion, based on their interactions with growers, that the potential of A. limonicus and A. degenerans may be somewhat lower that the previous paragraphs suggest, at least in vegetable crops in northern Europe. However, experimental data substantiating this view are not available.
Parasitic wasps. To date, two species of parasitoids, Ceranisus menes and C. americensis have been investigated to assess their potential to suppress WFT in greenhouse crops (Loomans et al. 1995b, Loomans and van Lenteren 1996). Neither was found to have any effect on thrips populations. Both species’ development times are long relative to that of WFT. As a consequence, parasitoid populations do not increase relative to thrips numbers and little or no control results. In Loomans and van Lenteren’s (1996) test of C. americensis, for example, parasitism in a rose house remained below 10% over a five month trial even though an initial release of 2000 wasps was made. Loomans and van Lenteren (1995) and Loomans and Murai (1997) review literature on known species of thrips parasitoids and make suggestions on potential species for further investigations. Research on tropical species of thrips and their parasitoids may be desirable, as these parasitoids may have relatively faster rates of development.
Biology and Efficacy of Pathogens
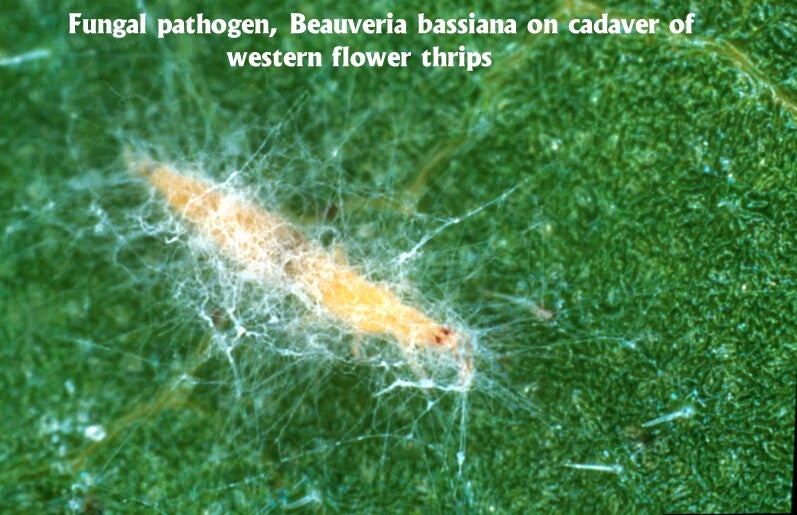
Fungi. While there are relatively few data available on pathogens of thrips, fungi are invariably the most common microbes recovered (see Butt & Brownbridge 1997 for a comprehensive review). This has prompted interest in the exploitation of these microorganisms for thrips control. Fungi do not have to be ingested to be infective; rather they are able to directly infect insects via the cuticle (Charnley et al. 1997). This "contact activity" makes them unique among insect pathogens, and they have a demonstrated capability to infect and suppress piercing-sucking insects. Isolates of Verticillium lecanii have been available commercially for many years in Europe for control of thrips and other glasshouse pests (Ravensberg et al. 1990, van der Schaaf et al. 1991, Helyer et al. 1992). More recently, products based on Beauveria bassiana have been registered for thrips control on ornamental, vegetable and nursery crops in the United States. Currently two Beauveria-based products, BotaniGard® (Mycotech Corp.) and Naturalis®-O (Troy Biosciences), are available for thrips management in greenhouses. Another fungal species, Paecilomyces fumosoroseus, is registered (Pfr-97) but is not presently available. All of these fungi can be readily produced on artificial substrates, making them ideal candidates for commercialization. Entomophthoralean fungi have also been isolated from WFT, and observed causing epizootics on greenhouse cucumbers (Montserrat et al. 1998). While these latter fungi are highly specific and often very virulent, they are very difficult to mass produce for use as biopesticides. The prospects for their commercialization are currently poor.
Insect-killing fungi possess many features that make them ideal for use in IPM and have several distinct advantages over other biological control agents: (1) their activity is unaffected by factors such as day length that can limit the activity of some thrips predators such as Orius spp. (van den Meiracker 1994); (2) they are active over a range of environmental conditions and can be effective in different climatic zones (Zimmermann 1994). They have great potential for inclusion in a thrips management program but wider use has been hindered by the view that they are less effective, less reliable, slower-acting, more difficult to apply, and have poorer shelf-life than their chemical counterparts. Advances have been made over the last decade, however, that have improved the quality, performance and price of these microbial insecticides, making them more effective and more cost-competitive with chemical pesticides (Georgis 1997). Like any other biological agent, they take longer to work than many chemical insecticides, but today’s formulations are stable and easily mixed for spray application using standard spray equipment. Furthermore, although high ambient humidity can enhance the infection process, it is not a pre-requisite to obtaining good infection and control; microclimate humidity is far more important in this regard (Clarkson & Charnley 1996). Also, through selection of appropriate strains and use of novel formulations, infection can occur even when ambient humidity is low (Zimmermann 1994, Charnley et al. 1997).
In order to utilize fungi efficiently, though, it is important to understand how they work, how they may best be applied, and places where they can and cannot be used. They are not effective against all pests or all developmental stages and will be best used as part of preventative, integrated crop management program. Interactions with other controls must be understood if fungi are to be integrated into IPM programs.
The "active ingredients" in fungal preparations are spores, also called conidia. These must contact the host to be effective, either directly at the time of spraying or later as the host moves over treated foliage. Good spray coverage is, therefore, essential. Once the spores are attached to the insect, they germinate and pierce the insect’s body wall. The fungus then multiplies within the body, causing the insect to stop feeding and die a few days later.
Invasion and development of V. lecanii in WFT have been studied by Schreiter et al. (1994). Vestergaard et al. (1995) and Brownbridge (1995) showed that B. bassiana, M. anisopliae and V. lecanii tend to be more active against WFT than are P. fumosoroseus or P. farinosus. Metarhizium anisopliae was found to be a more aggressive pathogen than V. lecanii, perhaps due to subtle differences in the mode of infection: V. lecanii often colonizes the host surface before invading the body, whereas M. anisopliae appears to penetrate and infect the insect soon after germination (Schreiter et al. 1994, Vestergaard et al. 1995). WFT mortality from fungal infection is dose-dependent; the more spores that contact the insect, the more rapid the kill and the higher the rate of infection (Brownbridge et al. 1994, Vestergaard et al. 1995). Temperature influences infection and different pathogens work best at different temperatures. Metarhizium anisopliae strains tested by Vestergaard et al. (1995) were most virulent at 73°F (23°C). A drop in temperature of just 5-9°F (3-5°C) increased the time of death by one day, which could be critical in heavily infested greenhouses.
Not all WFT developmental stages are equally susceptible to fungal infection. Eggs, for example, are laid in host plant tissue and are thus protected from contact with the pathogen. Larval and pupal stages are more resistant than adults, presumably because fungal inoculum is shed when the insects molt. Molting is an important factor in insect resistance to infection, especially in an insect like WFT in which the time between molts is short. Pupae are more susceptible to infection by M. anisopliae than are larvae (Vestergaard et al. 1995). Adults are the most susceptible stage; however, infected adults continue to lay eggs for a few days. Re-treatment of an infested crop is, therefore, usually necessary to ensure good control.
Verticillium lecanii has been used to successfully control WFT on chrysanthemums and cucumbers (van der Schaaf et al. 1991, Helyer et al. 1992). Infection rates of up to 60% were obtained on cucumber, even though the ambient humidity fell as low as 75%; higher doses of V. lecanii were used to compensate for the negative effects of low humidity on pathogen efficacy (van der Schaaf et al. 1991). Control of several insect pests on chrysanthemum was achieved when V. lecanii was applied every 14 days and relative humidity was artificially raised to high levels (> 95%) for four consecutive nights following spraying. Under these conditions, epizootics developed and controlled the insect populations with no adverse effects on the crop (Helyer et al. 1992). Good control of WFT has also been achieved with a commercial preparation of P. fumosoroseus (Pfr-97) (Lindquist 1996). Spray trials carried out in California, Maryland and Vermont have shown that B. bassiana (BotaniGard®) efficiently controlled thrips on roses, carnations and potted sunflower, and suppressed populations in chrysanthemums (Brownbridge et al. 1996, Gill 1997, Murphy et al. 1998). However, trials conducted in Texas with Botanigard® and Naturalis®-O on western flower thrips infesting potted chrysanthemums and African violets failed to detect efficacy for either product (Thompson et al. in press).
While in some cases three to five sprays at 3-5 day intervals have provided control of high populations, it is better to monitor thrips and begin fungal applications as soon as thrips are detected. This allows lower dose rates to be used and applications to be made less often.
Most WFT pupate in the soil and fungi can be applied to soil to kill this stage. Verticillium lecanii successfully infected WFT pupae in soil, but the fungus persisted poorly in non-sterile potting media (Hirte et al. 1994, Sermann et al. 1994). Brownbridge & Adamowicz (1995) showed that drench applications of P. fumosoroseus, B. bassiana and M. anisopliae effectively suppressed WFT in potting soil. In addition, B. bassiana and M. anisopliae inoculum levels actually increased over time, potentially providing a long-term control of thrips pupae in soil. Good control of WFT pupae in potting compost was also obtained by Helyer et al. (1995) using M. anisopliae, although better control was obtained when the fungus was applied before rather than after pupation. These studies show the potential for controlling WFT in the soil, but other plant management practices may affect the results. Some fertilization practices (such as use of fresh manure), for example, are detrimental to the survival of B. bassiana; other amendments (such as use of composted manure) promote multiplication of the fungus in soil (Rosin et al. 1996).
Fungal biopesticides must be held under appropriate conditions at all times - in transit and in storage on the farm. Fungi are sensitive to high temperatures and even warm conditions can be damaging if prolonged. Generally, if the products are kept cool (< 60°F [15°C]) and dry, quality will be maintained for at least a year. Short term storage (under two hours) at higher temperatures is unlikely to have a significant effect, but if fungi are held under warm conditions for extended periods, the spores are inactivated and the rate of germination in the survivors declines (Brownbridge 1996). In general, the higher the storage temperature, the more rapid the rate of inactivation. Temperatures >40°C are not uncommon in pesticide storage sheds and are unsuitable for fungi. Products can be rendered totally inactive within a few weeks or less at such temperatures.
Spore viability of fungal biopesticides is not reduced by most spray adjuvants, but growers should contact the biopesticide’s manufacturer to obtain an updated list of compatible adjuvants and recommendations for their use. Spray solutions should be used immediately because if they are held for several hours under warm conditions, spore viability will decline and the level of control will be reduced.
High-volume spray applications that produce a fine mist with good leaf wetting (but not to run-off) give better levels of control than low volume electrostatic sprays (Brownbridge et al. 1996).
Tank mixes of fungal biopesticides and other pesticides should only be prepared according to the manufacturer’s guidelines because some materials are harmful to fungi. Some older insecticides are inhibitory, and if used should be applied separately. Fungicides are the most inhibitory, and need to be used with caution in any spray program in which beneficial fungi are used. However, fungi may be applied successfully either 48-72 h before or after fungicides have been applied.
Fungal pathogens are often compatible with the other natural enemies. The fungi discussed here are compatible with the thrips predators Orius insidiosus and Amblyseius cucumeris, and honeybees (Brownbridge, pers. comm.). Similarly, the fungal pathogen Aschersonia aleyrodis is compatible with the parasitoid Encarsia formosa (used for whitefly control) because it does not infect whiteflies that have been parasitized for more than three days (Fransen and van Lenteren 1994).
Nematodes
Nematodes in the families Steinernematidae and Heterorhabditidae kill a wide range of insects they contact in moist habitats, such as soil. Several species in the genera Steinernema and Heterorhabditis have been commercialized successfully for control of insects in soil. Several nematodes in the genus Steinernema have been tested to assess their ability to kill WFT stages in soil. Results have varied, with 4 to 77% mortality in various tests (Tomalek 1991, 1994; Helyer et al. 1995; Chyzik et al. 1996). Tests with Heterorhabditis bacteriophora strain HP88 have shown 36 to 49% mortality (Chyzik et al. 1996). The problem inherent in the use of any of these nematodes is that only a small portion of the WFT population will be present in the soil at any one time. Also, some pupation takes place on the plant, where environmental conditions are not conducive to nematode infection. Because these nematodes have little or no effect in the habitat where adult and larval thrips are found (the plant’s foliage and buds), there is little prospect of these nematodes being highly effective, at least not without very frequent use.
In contrast to the above families of nematodes, members of the family Sphaerulariidae have a different mode of action that may be more suitable for use against WFT on foliage. The sphaerulariid Thripinema nicklewoodi was the most common natural enemy associated with WFT in California, the area from which WFT is believed to have originated (Heinz et al. 1996). Unlike steinernematid or heterorhabditid nematodes which quickly kill their hosts, T. nicklewoodi infects but does not kill thrips larvae. Rather, larvae live to become sterile adults that vector the nematodes (in their feces) into buds and flowers where thrips congregate. This biology suggests that T. nicklewoodi might be effectively transmitted within a WFT population on plants. While aspects of the biology of this species of nematode are known (Lysaght 1937, Nickle and Wood 1964, Wilson and Cooly 1972, Siddiqui 1985, Greene and Parrella 1995), greenhouse studies on population interactions with WFT are lacking.
Summary
Biological control options for western flower thrips are better developed for vegetables than ornamentals. On sweet peppers, a nondiapausing strain of A. cucumeris provides adequate control and is in commercial use. On cucumber, control is not reliable with A. cucumeris; however, use of either A. limonicus or A. degenerans provides effective control. Low cost rearing of A. degenerans on castor bean has been developed, as have banker plant systems for in-greenhouse rearing of this species.
Of the various Orius species tested for control of WFT, all but O. albidipennis appear to be inadequate during fall and winter because of diapause induced by short days which stops oviposition by females. Orius albidipennis seems not to enter diapause, even at very short daylength and may be the most suitable of the Orius species for fall and winter use.
Parasitoids attacking thrips do not show much potential for the control of WFT. Development of new biopesticides based on pathogenic fungi is progressing. Identification of better strains through screening and development of better formulation and application methods are likely to lead to increasingly more reliable biopesticides for WFT control in greenhouses.
Nematodes in the families Steinernematidae and Heterorhabditidae seem poorly suited for biological control of WFT because they are ineffective on foliage and can only be used against thrips in soil. Because the majority of the thrips are on foliage, control of thrips populations with these nematodes is not achieved. The sphaerulariid nematode T. nicklewoodi, not yet commercially available, appears to be better suited to infect thrips on foliage because of its unique method of spread in feces of infected, sterilized thrips.
References |
- Allen, W. R. and A. B. Broadbent. 1986. Transmission of tomato spotted wilt virus in Ontario greenhouses by the western flower thrips Frankliniella occidentalis (Pergande). Canadian Journal of Plant Pathology 8: 33-38.
- Allen, W. R. and J. A. Matteoni. 1991. Petunia as an indicator plant for use by growers to monitor for thrips carrying the tomato spotted wilt virus in greenhouses. Plant Disease 75: 78-82.
- Bailey, D.E. and R.F. Smith. 1956. The Frankliniella occidentalis (Pergande) complex in California (Thysanoptera: Thripidae). University of California Publications in Entomology 10: 359-410.
- Baker, J. R., J. A. Bethke, and E. A. Shearin. 1995. Insect screening, pp. 155-170 In Banner, W. and M. Klopmeyer (eds.). New Guinea Impatiens: a Ball Guide. Ball. Publishing, Batavia, Illinois, United States.
- Bautista, R. C., R. F. L. Mau, J. J. Cho, and D. M. Custer. 1995. Potential of tomato spotted wilt tospovirus plant hosts in Hawaii as virus reservoirs for transmission by Frankliniella occidentalis (Thysanoptera: Thripidae). Phytopathology 85: 953-958.
- Beshear, R.J., 1983. New records of thrips in Georgia. Journal of the Georgia Entomological Society 18: 342-344.
- Best, R. J. 1968. Tomato spotted wilt virus, pp. 65-145. In K. M. Smtih and M. A. Lauffer (eds.). Advances in Virus Research. Academic Press, New York.
- Bethke, J.A., R.A. Redak and T.D. Paine. 1994. Screens deny specific pests entry to greenhouses. California Agriculture 48(3): 37-40.
- Blumel, S. 1996. Effect of selected mass-rearing parameters on O. majusculus (Reuter) and O. laevigatus (Fieber). Bulltein IOBC/WPRS 19(1): 15-18.
- Brødsgaard, H. F. 1994a. Effect of photoperiod on the bionomics of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Journal of Applied Entomology 117: 498-507.
- Brødsgaard, H. F. 1994b. Insecticide resistance in European and African strains of western flower thrips (Thysanoptera: Thripidae) tested in a new residue-on-glass test. Journal of Economic Entomology 87: 1141-1146.
- Brownbridge, M. 1995. Prospects for mycopathogens in thrips management, pp. 281-295. In Parker B. L., M. Skinner and T. Lewis (eds.). Thrips Biology and Management. Plenum Press, New York.
- Brownbridge, M. 1996. Effect of storage temperature and formulation on viability and infectivity of Beauveria bassiana and Paecilomyces fumosoroseus, pp. 11. In Abstracts, Society for Invertebrate Pathology 29th Annual Meeting. 1-6 Sept, 1996, Cordoba, Spain.
- Brownbridge, M. and A. Adamowicz. 1995. Activity and persistence of thrips mycopathogens in potting soil, pp. 10. In Abstracts, Society for Invertebrate Pathology 28th Annual Meeting, 16-21 July 1995, Ithaca, New York.
- Brownbridge, M., R. A. Humber, B. L. Parker, and M. Skinner. 1993. Fungal entomopathogens recovered from Vermont forest soils. Mycologia 85: 358-361.
- Brownbridge, M., D.L. McLean, B.L. Parker and M. Skinner. 1994. Use of fungal pathogens for insect control in greenhouses, pp. 7-20. In Robb, K. (ed.). Proceedings, Tenth Conference on Insect and Disease Management on Ornamentals. 19-21 Feb. 1994, Dallas, Texas. Ball Publishing, Batavia, Illinois.
- Brownbridge, M., A. Adamowicz, M. Skinner and B.L. Parker. 1996. Management of silverleaf whitefly and western flower thrips with Beauveria bassiana: effect of spray techniques on efficacy, pp. 11-12. In Abstracts, Society for Invertebrate Pathology 29th Annual Meeting. 1-6 Sept, 1996, Cordoba, Spain.
- Butt, T. and M. Brownbridge. 1997. Fungal pathogens of thrips, pp. 399-433. In Lewis, T. (ed). Thrips as Crop Pests. CAB International, Wallingford, United Kingdom.
- Castañé, C. and F. G. Zalom. 1994. Artificial oviposition substrate for rearing Orius insidiosus (Hemiptera: Anthocoridae). Biological Control 4: 88-91.
- Castineiras, A., R. M. Baranowski and H. Glenn. 1996. Temperature response of two strains of Ceranisus menes (Hymenoptera: Eulophidae) reared on Thrips palmi (Thysanoptera: Thripidae). Florida Entomologist 79: 13-20.
- Chambers, R. J., S. Long, and B. L. Helyer. 1993. Effectiveness of Orius laevigatus (Hem.: Anthocoridae) for the control of Frankliniella occidentalis on cucumber and pepper in the United Kingdom. Biocontrol Science and Technology 3: 295-307.
- Charnley, A.K., B. Cobb and J.M. Clarkson. 1997. Towards the improvement of fungal insecticides, pp. 115-126. In H.F. Evans [ed.]. Microbial Insecticides: Novelty or Necessity? Proc. 1997 British Crop Protection Council Symposium (No. 68.). BCPC, United Kingdom.
- Chyzik, R., I. Glazer, and M. Klein. 1996. Virulence and efficacy of different entomopathogenic nematode species against western flower thrips (Frankliniella occidentalis). Phytoparastica 24: 103-110.
- Cho, J.J., R.F.L. Mau, D. Gonsalves and W.C. Mitchell. 1986. Reservoir weed hosts of tomato spotted wilt virus. Plant Disease 70(11): 1014-1017).
- Cho, J. J., R. F. L. Mau, R. T. Hamasaki, and D. Gonsalves. 1988. Detection of tomato spotted wilt virus in individual thrips by enzyme-linked immunosorbent assay. Phytopathology 78: 1348-1352.
- Clarkson, J.M. & A.K. Charnley. 1996. New insights into the mechanisms of fungal pathogenesis in insects. Trends in Microbiology 4: 197-204.
- Coll, M. and R. L. Ridgeway. 1995. Functional and numerical responses of Orius insidiosus (Heteroptera: Anthocoridae) to its prey in different vegetable crops. Annals of the Entomological Society of America 88: 732-738.
- Daughtrey, M. L. 1996. Detection and identification of tospoviruses in greenhouses. Acta Horticulturae 431: 90-98.
- Daughtrey, M. L., R. L. Wick, and J. L. Peterson. 1995. Compendium of Flowering Potted Plant Diseases. APS Press, St. Paul, Minnesota, United States.
- Daughtrey, M. L., R. K. Jones, J. W. Moyer, M. E. Daub, and J. R. Baker. 1997. Tospoviruses strike the greenhouse industry: INSV has become a major pathogen on flower crops. Plant Disease 81: 1220-1230.
- Ferguson, G. M. and J. S. Schmidt. 1996. Effect of selected cultivars on Orius insidiosus. Bulletin IOBC/WPRS 19(1): 39-42.
- Fery, R. L. and J. M. Schalk. 1991. Resistance in pepper (Capsicum annuum L. ) to western flower thrips (Frankliniella occidentalis [Pergande]). HortScience 26: 1073-1074.
- Fransen, J. J. and J. Tolsma. 1992. Releases of the minute pirate bug, Orius insidiosus (Say) (Hemiptera: Anthocoridae), against western flower thrips, Frankliniella occindentalis (Pergande) on chrysanthemum. Mededlingen van de Faculteit Landbouwwetenschappen Rijksuniversiteit Gent 57 (2b): 479-484.
- Fransen, J. J. and J.C. van Lenteren. 1994. Survival of the parasitoid Encarsia formosa after treatemnt of parasitized greenhouse whitefly larvae with fungal spores of Aschersonia aleyrodis. Entomologia Experimentalis et Applicata 71: 235-243.
- Frescata, C. and A. Mexia. 1996. Biological control of thrips (Thysanoptera) by Orius laevigatus (Heteroptera: Anthocoridae) in organically-grown strawberries. Biological Agriculture and Horticulture 13 (2): 141-148.
- Frey, J. E., R. V. Cortada, and H. Helbling. 1994. The potential of flower odours for use in population monitoring of western flower thrips, Frankliniella occidentalis (Perg.) (Thysanopera: Thripidae). Biocontrol Science and Technology 4: 177-186.
- Gaum, W. G., J. H. Giliomee, and K. L. Pringle. 1994. Life history and life tables of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), on English cucumber. Bulletin of Entomological Research 84: 219-224.
- Georgis, R. 1997. Commercial prospects of microbial pesticides in agriculture, pp. 243-252. In H.F. Evans [ed.]. Microbial Insecticides: Novelty or Necessity? Proceedings, 1997 British Crop Protection Council Symposium (No. 68.). BCPC, United Kingdom.
- Gerin, C., Th Hance and G. van Impe, 1994. Demographical parameters of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Journal of Applied Entomology 118: 370-377.
- German, T. L., D. E. Ullman, and J. W. Moyer. 1992. Tospoviruses: diagnosis, molecular biology, phylogeny, and vector relationships. Annual Review of Phytopathology 30: 315-348.
- Gilkeson, L. A. W. D. Morewood and D. E. Elliot. 1990. Current status of biological control of thrips in Canadian greenhouses with Amblyseius cucumeris and Orius tristicolor. IOBC/WPRS Bulletin 13: 71-75.
- Gill, S. 1994. Thrips management and biological control. GrowerTalks 58 (6): 36-40.
- Gill, S. 1997. You can control thrips biologically. Grower Talks 61 (July): 114-117.
- Goodwin, S. and M. Y. Steiner. 1996. Survey of Australian native natural enemies for control of thrips. Bulletin IOBC/WPRS 19(1): 47-50.
- Greene, I. D. and M. P. Parrella. 1995. Two new natural enemies of western flower thrips in California, pp. 277-283. In Parker, B. L., M. Skinner, and T. Lewis (eds.). Thrips Biology and Management. NATO ASI Series., Series-A: Life Sciences, Vol. 276.
- Heinz, F., M. P. Parrella, and J. P. Newman. 1992. Time-efficient use of yellow sticky traps in monitoring insect populations. Journal of Economic Entomology 85: 2263-2269.
- Heinz, K. M., L. M. Heinz, and M. P. Parrella. 1996. Natural enemies of western flower thrips indigenous to California ornamentals. IOBC/WPRS Bulletin 19 (1): 51-54.
- Helyer, N. L. and P. J. Brobyn. 1992. Chemical control of western flower thrips (Frankliniella occidentalis [Pergande]). Annals of Applied Biology 121: 219-231.
- Helyer, N., G. Gill, A. Bywater & R. Chambers. 1992. Elevated humidities for control of chrysanthemum pests with Verticillium lecanii. Pesticide Science 36: 373-378.
- Helyer, N. L., P. J. Brobyn, P. N. Richardson, and R. N. Edmondson. 1995. Control of western flower thrips (Frankliniella occidentalis [Pergande]) pupae in compost. Annals of Applied Biology 127: 405-412.
- Hessein, N. A. and M. P. Parrella. 1990. Predatory mites help control thrips on floriculture crops. California Agriculture 44: 19-21.
- Higgins, C.J. and J.H. Myers, 1992. Sex ratio patterns and population dynamics of western flower thrips (Thysanoptera: Thripidae). Environmental Entomology, 21: 322-330.
- Hirte, W., H. Triltsch & H. Sermann. 1994. Growth and survivability of the entomopathogenic fungus Verticillium lecanii in the soil, pp. 226-229. In Smits, P.H. (ed.). Proceedings, Fourth European Meeting on Microbial Control of Pests. IOBC Working Group on Insect Pathogens and Insect Parasitic Nematodes, Zurich, Switzerland, 1993. IOBC Bull. 17.
- Hunter, C. D. 1997. Suppliers of Beneficial Organisms in North America. California Environmental Protection Agency, Department of Pesticide Regulation, 1020 N. St., Rm. 161, Sacramento , CA, 95814-5624 (ph = 916-324-4100).
- Immaraju, J. A., T. D. Paine, J. A. Bethke, K. L. Robb., and J. P. Newman. 1992. Western flower thrips (Thysanoptera: Thripidae) resistance to insecticides in coastal California greenhouses. Journal of Economic Entomology 85: 9-14.
- de Jager, C. M. and R. P. T. Butot 1992. Susceptibility of Chrysanthemum cultivars to thrips (Frankliniella occidentalis) infestation and the role of some physical plant characters. Ser-Entomol. 49: 263-264.
- de Jager, C. M., R. P. T. Butot, P. G. L. Klinkhamer, T. J. de Jong, K. Wolff, and E. van der Meijden. 1995. Genetic variation in chrysanthemum for resistance to Frankliniella occidentalis. Entomologia Experimentalis et Applicata 77: 277-287.
- Katayama, H., 1997. Effect of temperature on development and oviposition of western flower thrips Frankliniella occidentalis (Pergande). Japanese Journal of Applied Entomlogy and Zoology, 41: 225-231 (in Japanese with English summary).
- de Klerk, M. L. and P. M. J. Ramakers. 1986. Monitoring population densities of the phytoseiid predator Amblyseius cucumeris and its prey after large scale introductions to control Thrips tabaci on sweet pepper. Mededelingen van de Faculteit Landbouwwetenschappen Rijksuniversiteit Gent 51 (3a): 1045-1048.
- de Kogel, W.J., M van der Hoek, C. Mollema, 1997. Variation in performance of western flower thrips populations on susceptible and partially resistant cucumber. Entomologia Experimentalis et Applicata, 83: 73-80.
- Kostiainen, T. S. and M. A. Hoy. 1996. The Phytoseiidae as Biological Control Agents of Pest Mites and Insects: A Bibliography. Monograph 17, Department of Entomology and Nematology, University of Florida, Gainesville, FL.
- Kumar, N. K. K., D. E. Ullman, and J. J. Cho. 1995. Resistance among Lycopersicon species to Frankliniella occidentalis (Thysanoptera: Thripidae). Journal of Economic Entomology 88: 1057-1065.
- Lewis, T. (ed.), 1997. Thrips as Crop Pests. CAB International, United Kingdom.
- Lindquist, R. 1996. Microbial control of greenhouse pests using entomopathogenic fungi in the USA. IOBC Bull. 19: 153-156.
- Loomans, A.J.M. 1991. Collection and first evaluation of hymenopterous parasites of thrips as biological control agents of Frankliniella occidentalis. SROP/WPRS Bulletin 14: 73-82.
- Loomans, A. J. M. and J. C. van Lenteren. 1995. Biological control of thrips pests: a review on thrips parasitoids, pp. 88-201. In Loomans, A. J. M., J. C. van Lenteren, M. G. Tommasini, S. Maini, and J. Riudavets (eds.). Biological Control of Thrips Pests. Wageningen Agricultural University Papers 95-1, printed by Veenman Drukkers, Wageningen, The Netherlands.
- Loomans, A. J. M. and J. C. van Lenteren. 1996. Prospects of Ceranisus americensis (Girault) (Hymenoptera: Eulophidae) as a potential biological control agent of thrips pests in protected crops. SROP/WPRS Bulletin 19: 95-98.
- Loomans, A. J. M. and T. Murai, 1997. Culturing thrips and parasitoids, pp. 477-503. In Lewis, T. (ed.) Thrips as Crop Pests. CAB International, United Kingdom.
- Loomans, A. J. M., T. Murai, J. P. N. F. van Heest, and J. C. van Lenteren. 1995a. Ceranisus menes (Hymenoptera: Eulophidae) for control of western flower thrips: biology and behavior, pp. 263-268. In Parker, B. L., M. Skinner, and T. Lewis (eds.). Thrips Biology and Management. NATO ASI Series., Series-A: Life Sciences, Vol. 276.
- Loomans, A.J.M., J. Tolsma, J.P.N.F. van Heest and J.J. Fransen, 1995b. Releases of parasitoids (Ceranisus spp.) as biological control agents of western flower thrips (Frankliniella occidentalis) in experimental greenhouses. Mededelingen Faculteit Landbouwwetenschappen, Rijksuniversiteit Gent 60/3a: 869-877.
- Loomans, A. J. M., T. Murai and I.D. Greene, 1997. Interactions with hymenopterous parasitoids and parasitic nematodes, pp. 355-397. In Lewis, T. (ed.) Thrips as Crop Pests. CAB International, United Kingdom.
- Lysaght, A. M. 1937. An ecological study of a thrips (Aptinothrips rufis) and its nematode parasite (Anguillulina aptini). Journal of Animal Ecology 6: 169-192.
- Montserrat, M., C. Castañé & S. Santamaria. 1998. Neozygites parvispora (Zygomycotina: Entomophthorales) causing an epizootic in Frankliniella occidentalis (Thysanoptera: Thripidae) on cucumber in Spain. Journal of Invertebrate Pathology 71: 165-168.
- Morewood, W. D. and L. A. Gilkeson. 1991. Diapause induction in the thrips predator Amblyseius cucumeris under greenhouse conditions. Entomophaga 36: 253-263.
- Mound, L. A. and G. Kibby. 1998. Thysanoptera, an Identification Guide, 2nd edition. CAB International, United Kingdom.
- Murphy, B.C., T.A. Morisawa, J.P. Newman, S.A. Tjosvold & M.P. Parrella. 1998. Fungal pathogen provides control of western flower thrips in greenhouse flowers. California Agriculture (in press).
- Nasruddin, A. and D. R. Smitley. 1991. Relationship of Frankliniella occidentalis (Thysanoptera: Thripidae) population density and feeding injury to the frequency of insecticide applications to gloxinia. Journal of Economic Entomology 84: 1812-1817.
- Nickle, W. R. and G. W. Wood. 1964. Howardula aptini (Sharga 1932) parasite in blueberry thrips in New Brunswick. Canadian Journal of Zoology 42: 843-846.
- Oetting, R. D. 1988. Application of abamectin for Frankliniella occidentalis control in glasshouses. Proceedings of the British Crop Protection Conference on Pests and Diseases, London, British Crop Protection Council 1: 181-185.
- Parker, B. L., M. Skinner, and T. Lewis (eds.). 1995. Thrips Biology and Management. NATO ASI Series. Series A: Life Sciences Vol. 276.
- Parrella, M. P. 1995. Thrips management guide part II. Proper identification. GrowerTalks 59 (July): 82-94.
- Parrella, M. P. and B. Murphy. 1996. Western flower thrips: identification, biology, and research on the development of control strategies, pp. 115-118. Bulletin IOBC/WPRS 19 (1): 115-118.
- Ramakers, P. M. J. 1995. Biological control using oligophagous predators, pp. 225-229. In Parker, B. L., M. Skinner, and T. Lewis (eds.) Thrips Biology and Management. Plenum Press, New York.
- Ramakers, P. M. J. and S. J. P. Voet. 1995. Use of castor bean, Ricinus communis, for the introduction of the thrips predator Amblyseius degenerans on glasshouse-grown sweet peppers. Mededelingen Faculteit Landbouwwetenschappen, Rijksuniversiteit Gent 60/3a: 885-891.
- Ramakers, P. M. J. and S. J. P. Voet. 1996. Introduction of Amblyseius degenerans for thrips control in sweet peppers with potted castor beans as banker plants, pp. 127-130. Bulletin IOBC/WPRS 19 (1): 127-130.
- Ravensberg, W. J. and K. Altena. 1987. Recent developments in the control of thrips in sweet pepper and cucumber. Bulletin SROP 10 (2): 160-164.
- Ravensberg, W. J., M. Malais, and D. A. van der Schaaf. 1990. Verticillium lecanii as a microbial insecticide against glasshouse whitefly, pp. 265-268. In Brighton Crop Protection Conference--Pests and Disease British Crop Protection Council, The Lavenham Press, Ltd., Lavenham, United Kingdom.
- Rice, D. J., T. L. German, R. F. L. Mau and F. M. Fujimoto. 1990. Dot blot detection of tomato spotted wilt virus RNA in plant and thrips tissues by cDNA clones. Plant Disease 74: 274-276.
- Riudavets, J. 1995. Predators of Frankliniella occidentalis (Perg.) and Thrips tabaci Lind.: a review, pp. 43-87. In Loomans, A. J. M., J. C. van Lenteren, M. G. Tommasini, S. Maini, and J. Riudavets (eds.). Biological Control of Thrips Pests. Wageningen Agricultural University Papers 95-1, printed by Veenman Drukkers, Wageningen, The Netherlands.
- Riudavets, J. and C. Castañé. 1998. Identification and evaluation of native predators of Frankliniella occidentalis (Thysanoptera: Thripidae) in the Mediterranean. Environmental Entomology 27: 86-93.
- Robb, K. L. 1989. Analysis of Frankliniella occidentalis (Pergande) as a pest of floriculutral crops in California greenhouses. Ph.D. Dissertation, University of California, Riverside.
- Robb, K.L. and M. P. Parrella. 1995. IPM of western flower thirps, pp. 365-370. In Parker, B. L., M. Skinner, and T. Lewis (eds.) Thrips Biology and Management. Plenum Press, New York.
- Robb, K. L., J. Newman, J. K. Virzi and M. P. Parrella. 1995. Insecticide resistance in western flower thrips, pp. 341-346. In Parker, B. L., M. Skinner, and T. Lewis (eds.). Thrips Biology and Management. NATO ASI Series., Series-A: Life Sciences, Vol. 276.
- Rodriguez-Reina, J. M., F. Ferragut, A. Carnero and M. A. Peña. 1994. Diapause in the predacious mites Amblyseius cucumeris (Oud.) and Amblyseius barkeri (Hug.): Consequences of use in integrated control programmes. Journal of Applied Entomology 118: 44-50.
- Rosin, F., D.I. Shapiro and L.C. Lewis. 1996. Effects of fertilizers on the survival of Beauveria bassiana. Journal of Invertebrate Pathology 68: 194-195.
- Rubin, A., O. Ucko, N. Orr, and R. Offenbach. 1996. Efficacy of natural enemies of the western flower thrips, Frankliniella occidentalis, in pepper flowers in the Arava Valley, Israel, pp. 139-142. Bulletin IOBC/WPRS 19 (1) 139-142.
- Sabelis, M.W. and P.C.J. van Rijn, 1997. Predation by insects and mites, pp. 259-354 In Lewis, T. (ed.) Thrips as Crop Pests. CAB International, United Kingdom.
- Sakimura, K. 1962a. Frankliniella occidentalis (Thysanoptera: Thripidae), a vector of the tomato spotted wilt virus with special reference to the color forms. Annals of the Entomological Society of America 55: 387-389.
- Sakimura, K. 1962b. Present status of thrips borne viruses, pp. 33-40. In editors Biological Transmission of Disease Agents. Academic Press, New York.
- Schmidt, M. E. and J. E. Frey. 1995. Monitoring of the western flower thrips, Frankliniella occidentalis, in greenhouses. Mededlingen van de Faculteit Landbouwwetenschappen Rijksuniversiteit Gent 60: 847-850.
- Schreiter, G., T. M. Butt, A. Beckett, S. Vestergaard, and G. Mortiz. 1994. Invasion and development of Verticillium lecanii in the western flower thrips, Frankliniella occidentalis. Mycological Research 98: 1025-1034.
- Sermann, H., U. Kästner and W. Hirte. 1994. Effectiveness of a soil application of Verticillium lecanii on soilborne stages of Frankliniella occidentalis, pp. 230-233. In Smits, P.H. (ed.). Proceedings, Fourth European Meeting on Microbial Control of Pests. IOBC Working Group on Insect Pathogens and Insect Parasitic Nematodes, Zurich, Switzerland, 1993. IOBC Bull. 17.
- Shipp, J.L. and N. Zariffa. 1991. Spatial patterns of and sampling methods for western flower thrips (Thysanoptera: Thripidae) on greenhouse sweet pepper. Canadian Entomologist 123: 989-1000.
- Shipp, J. L., M. R. Binns, X. Hao, and K. Wang. 1998. Economic injury levels for western flower thrips (Thysanoptera: Thripidae) on greenhouse sweet pepper. Journal of Economic Entomology 91: 671-677.
- Siddiqui, M. F. 1985. Tylenchida, Parasites of Plants and Insects. CAB Commonwealth Institute of Parasitology. London, United Kingdom.
- Smitley, D. R. 1992. Biological control of western flower thrips with Orius, a predaceous bug. Roses Inc. Bulletin 99 (April): 59-64.
- Soria, C. and C. Mollema. 1992. Effects of resistance in cucumber upon life-history components of Frankliniella occidentalis. Ser-entomol. 49: 265-266.
- Steiner, M. Y. and A. J. Tellier. 1990. Western flower thrips, Frankliniella occidentalis (Pergande), in greenhouse cucumbers in Alberta, Canada. IOBC/WPRS Bulletin 13 (5): 202-205.
- Stobbs, L. W., A. B. Broadbent, W. R. Allen, A. L. Stirling. 1992. Transmission of tomato spotted wilt virus by the western flower thrips to weeds and native plants found in southern Ontario. Plant Disease 76: 23-29.
- Thompson, S., P. C. Krauter, and K. M. Heinz. 1998. Use of the fungus Beauveria bassiana as a management tool for greenhouse pests. Greenhouse Grower in press.
- Tomalak, M. 1991. Selection of a nematode, Steinernema feltiae, for control of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). IOBC/WPRS Bulletin 12: 29-66.
- Tomalak, M. 1994. Genetic improvement of Steinernema feltiae for integrated control of the western flower thrips, Frankliniella occidentalis. IOBC/WPRS Bulletin 17: 17-20.
- Tommasini, M. G. and S. Maini. 1995. Frankliniella occidentalis and other thrips harmful to vegetable and ornamental crops in Europe, pp. 1-42. In Loomans, A. J. M., J. C. van Lenteren, M. G. Tommasini, S. Maini, and J. Riudavets (eds.). Biological Control of Thrips Pests. Wageningen Agricultural University Papers 95-1, printed by Veenman Drukkers, Wageningen, The Netherlands.
- Tommasini, M. G. and G. Nicoli. 1996. Evaluation of Orius spp. as biological control agents of thrips pests. Further experiments on the existence of diapause in Orius laevigatus. Bulletin IOBC/WPRS 19 (1): 183-186.
- Trichilo, P. J. and T. F. Leigh, 1988. Influence of resource quality on the reproductive fitness of flower thrips (Thysanoptera: Thripidae). Annals of the Entomological Society of America 81: 64-70.
- Ullman, D.E., J.L. Sherwood and T.L. German. 1997. Thrips as vec tors of plant pathogens, pp. 539-565. In Lewis, T. (ed.) Thrips as Crop Pests. CAB International, United Kingdom.
- van Houten, Y. M. 1996. Biological control of western flower thrips on cucumber using the predatory mites Amblyseius cucumeris and A. limonicus, pp. 59-62. Bulletin IOBC/WPRS 19 (1): 59-62.
- van Houten, Y. M. and P. van Stratum. 1995. Control of western flower thrips on sweet pepper in winter with Amblyseius cucumeris (0udemans) and A. degenerans Berlese, pp. 245-248. In Parker, B. L., M. Skinner, and T. Lewis (eds.) Thrips Biology and Management. Plenum Press, New York.
- van Houten, Y. M., P. C. J. van Rijn, L. K. Tanigoshi, and P. van Stratum. 1995a. Preselection of predatory mites to improve year-round biological control of western flower thrips in greenhouse crops. Entomologia Experimentalis et Applicata 74: 225-234.
- van Houten, Y. M., P. van Stratum, J. Bruin, and A. Veerman. 1995b. Selection for non-diapause in Amblyseius cucumeris and Amblyseius barkeri and exploration of the effectiveness of selected strains for thrips control. Entomologia Experimentalis et Applicata 77: 289-295.
- van den Meiracker, R.A.F. 1994. Induction and termination of diapause in Orius predatory bugs. Entomologia Experimentalis et Applicata 73: 127-137.
- van den Meiracker, R.A.F. and P.M.J. Ramakers. 1991. Biological control of the western flower thrips Frankliniella occidentalis, in sweet pepper, with the anthocorid predator Orius insidiosus. Mededelingen Faculteit Landbouwwetenschappen, Rijksuniversiteit Gent 56/2a: 241-249.
- van der Schaaf, D.A., W.A. Ravensberg and M. Malais. 1991. Verticillium lecanii as a microbial insecticide against whitefly, pp. 120-123. In Proceedings of the 3rd European Meeting on Micro-bial Control of Pests. IOBC Working Group on Insect Pathogens and Insect Parasitic Nematodes, 24-27 Feb. 1991. Wageningen, Netherlands.
- van de Wetering, F., R. Goldbach and D. Peters. 1996. Transmission of tomato spotted wilt virus by Frankliniella occidentalis after viral acquisition during the first larval stage. Acta Horticulturae 431: 350-358.
- van Rijn, P. C. J., C. Mollema, and G. M. Steenhuis-Broers. 1995. Comparative life history studies of Frankliniella occidentalis and Thrips tabaci (Thysanoptera: Thripidae) on cucumber. Bulletin of Entomological Research 85: 285-297.
- van de Veire, G. Smagghe and D. Degheele. 1996. Laboratory test method to evaluate the effect of 31 pesticides on the predatory bug, Orius laevigatus (Het.: Anthocoridae). Entomophaga 41: 235-243.
- van de Wetering, F., R. Goldbach and D. Peters. 1996a. Transmission of tomato spotted wilt virus by Frankliniella occidentalis after viral acquisition during the first larval stage. Acta Horticulturae 431: 235-243.
- van de Wetering, F., J. Hulshof, F.R. van Dijken, R. Goldbach and D. Peters, 1996b. Differences in virus transmission and scar porduction among males and females of the western flower thrips. Proceedings of the Section Experimental and Applied Entomology of the Netherlands Entomological Society 7: 183-189.
- Vernon, R. S. and D. R. Gillespie. 1995. Influence of trap shape, size, and background color on captures of Frankliniella occidentalis (Thysanoptera: Thripidae) in a cucumber greenhouse. Journal of Economic Entomology 88: 288-293.
- Vestergaard, S., A. T. Gillespie, T. M. Butt, G. Schreiter, and J. Eilenberg. 1995. Pathogenicity of the hyphomycete fungi Verticillium lecanii and Metarhizium anisopliae to the western flower thrips, Frankliniella occidentalis. Biocontrol Science and Technology 5: 185-192.
- Wijkamp, I., J. van Lent, R. Kormelink, R. Goldbach and D. Peters. 1993. Multiplication of tomato spotted wilt virus in its insect vector, Frankliniella occidentalis. J. Gen. Virol. 74: 341-349.
- Wilson, T. H. and T. A. Cooley. 1972. A chalcidoid planidium and an entomopathogenic nematode associated with the western flower thrips. Annals of the Entomological Society of America 65: 414-418.
- Yudin, L. S., J. J. Cho, and W. C. Michell. 1986. Host range of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), with special reference to Leucaena glauca. Environmental Entomology 15: 1292-1295.
- Zhao, G. Y., W. Liu, J. M. Brown, and C. O. Knowles. 1995. Insecticide resistance in field and laboratory strains of western flower thrips (Thysanoptera: Thripidae). Journal of Economic Entomology 88: 1164-1170.
- Zimmermann, G. 1994. Strategies for the utilization of entomopathogenic fungi, pp. 67-73, In Proceedings of the 6th International Colloquium on Invertebrate Patholology and Microbial Control. 28 Aug-2 Sept 1994, Montpelier, France.
Additional Information |
Financial support for this publication was provide by the Massachusetts IPM Program and a grant from the New England Greenhouse Conference. We also thank Dr. D. Peters (Dept. of Virology, Wageningen, the Netherlands) for information on virus transmission and Peter Krauter for assistance in editing and Sean Werle for redrawing Figures 1 and 2. Special thanks are extended to all who contributed photographs.
Photograph Credits
- R. G. Van Driesche, Department of Entomology, University of Massachusetts, Amherst, MA, 01003.
- K. M. Heinz, Department of Entomology, Texas A and M University, College Station, Texas, 77843.
- J. C. van Lenteren, Department of Entomology, PO Box 8031, 6700 EH Wageningen, The Netherlands.
- A. Loomans, Department of Entomology, PO Box 8031, 6700 EH Wageningen, The Netherlands.
- R. Wick, Department of Microbiology, University of Massachusetts, Amherst, MA, 01003.
- T. Smith, Massachusetts Extension, University of Massachusetts, Amherst, MA, 01003.
- P. Lopes, Massachusetts Extension, University of Massachusetts, Cranberry Exp. Sta., E. Wareham, MA, 02538.
- J. P. Sanderson, Department of Entomology, Cornell University, Ithaca, NY, 14853.
- M. Daughtrey, Department of Plant Pathology, Cornell University, Long Island Horticultural Research Laboratory, Riverhead, NY, 11901.
- M. Brownbridge, Entomology Research Laboratory, University of Vermont, P.O. Box 53400, Burlington, VT, 05405.
Tables
Table 1. Summary of Life Table Data for Western Flower Thrips
|
Temperature |
Net Reproduction (Ro) on Cucumber | Net Reproduciton (Ro) on Chrysanthenum & Pollen | Developmental Time (days) on Cucumber | Developmental Time (days) on Chrysanthenum | % Females on Cucumber | Degree Days (egg to adult) on Cucmber | Degree Days (egg to adult) on Chrysanthenum |
| 15oC or 59oF | 1.02 | 42.25 | 48.0 | 39.1 | 55 | 274 | 333 |
|---|---|---|---|---|---|---|---|
| 18oC or 64oF | 2.54 | - | 30.7 | - | 58 | 253 | - |
| 20oC or 68oF | 5.00 | 86.49 | 25.2 | 26.1 | 61 | 242 | 472 |
| 23oC or 73oF | 5.77 | - | 18.9 | - | 69 | 228 | - |
| 23oC or 73oF | 5.77 | - | 18.9 | - | 69 | 228 | - |
| 25oC or 77oF | 6.04 | 99.51 | 12.8 | 12.9 | 64 | 245 | 403 |
| 30oC or 86oF | 8.48 | 35.39 | 10.1 | 9.3 | 86 | 257 | 347 |
| 35oC or 95oF | - | 2.69 | - | 10.7 | - | - | 439 |
Table 2. Potential Natural Enemies for Biological Control of Western Flower Thrips
| Natural Enemy Type | Commercially Available in Europe? | Commercially Available in North America? | WFT Lifestage Attacked | Factors Influencing Effectiveness | Target Crop |
|---|---|---|---|---|---|
| Predacious Bugs | |||||
| Dicyphus tamaninii | No | No | Larvae | Expensive to rear at high densities. Phytophagous | Vegetables, mainly peppers |
| Macrolophus caliginosus | Yes | No | Larvae | Expensive to rear at high densities. Phytophagous | Tomatoes and cucumbers. Used mainly for whitefly control |
| Orius laevigatus | Yes | No | Larvae and Adults | Needs long days (> 12 hrs) to reproduce | Tomatoes and cucumbers. Used mainly for whitefly control |
| Orius majusculus | Yes | No | Larvae and Adults | Needs long days (> 16 hrs) to reproduce | Tomatoes and cucumbers. Used mainly for whitefly control |
| Orius insidiosus | Yes | Yes | Larvae and Adults | Needs long days (> 12 hrs) to reproduce | Tomatoes and cucumbers. Used mainly for whitefly control |
| Orius albidipeninis | Yes | No | Larvae and Adults | - | Tomatoes and cucumbers. Used mainly for whitefly control |
| Predacious Mites | |||||
| Amblyseius cucumeris | Yes | Yes | Larvae | Needs long days (<11 hrs) to reproduce. Use of supplemental lighting to extend daylength. Can use non-diapausing strain. Needs pollen as alternate food | Sweet peppers |
| Amblyseius barkeri | No | No | Larvae | - | Naturally occurring in Europe |
| Amblyseius degenerans | Yes | Yes | Larvae | - | Pollen producing crops, e.g., sweet pepper |
| Amblyseius limonicus | No | No | Larvae | - | Sweet pepper |
| Parasitic Wasps | |||||
| Thripobius semiluteus | No | Yes | Does not attack WFT | Effective against greenhouse thrips | - |
| Ceranisus menes | No | No | Parasitizes WFT larvae | Slow development and a lack of pest control | - |
| Ceranisus americensis | No | No | Parasitizes WFT larvae | Slow development and a lack of pest control | - |
| Pathogenic Fungi | |||||
| Verticillium lecanii | Yes | No | WFT eggs not affected; WFT adults very susceptible; WFT larvae intermediate susceptibility | Requires good coverage and high humidity to be effective | Used mainly against whiteflies and to supplement other thrips control programs |
| Beanveria bassiana | No | Yes | WFT eggs not affected; WFT adults very susceptible; WFT larvae intermediate susceptibility | Requires good coverage and high humidity to be effective | Ornamentals and vegetables |
| Paecilomyces fumosoroseus | No | No | WFT eggs not affected; WFT adults very susceptible; WFT larvae intermediate susceptibility | Requires good coverage and high humidity to be effective | - |
| Metarhizium anisopliae | No | No | WFT eggs not affected; WFT adults very susceptible; WFT larvae intermediate susceptibility | Requires good coverage and high humidity to be effective | - |
| Parasitic Nematodes | |||||
| Steinernema carpocapsae | Yes | Yes | Propupae and pupae are susceptible to infection | Only effective against thrips in soil | All cropping systems |
| Steinernema feltiae | Yes | Yes | Propupae and pupae are susceptible to infection | Only effective against thrips in the soil | All cropping systems |
| Heterorhabditis | Yes | Yes | Propupae and pupae are susceptible to infection | Only effective against thrips in the soil | All cropping systems |
| Thripinema nicklewoodii | No | No | Infects larval stages and adult stage is sterilized. | Can only be reared in live hosts | Not currently used |