The Biology and Management of the Silverleaf Whitefly, Bemisia argentifolii Bellows and Perring (Homoptera: Aleyrodidae) on Greenhouse Grown Ornamentals
Prepared by Mark Hoddle, Extension Specialist and Director of Center for Invasive Species Research
mark.hoddle@ucr.edu
Introduction |
| Figure 1. Greenhouse whitefly adults |
The major foliar pest of poinsettia in U.S. greenhouses is the silverleaf whitefly, Bemisia argentifolii. This insect first became a greenhouse pest in the U.S. in 1986 when it became more common than the greenhouse whitefly (Trialeurodes vaporariorum), the primary whitefly pest of poinsettia at that time. The current problem with B. argentifolii began in 1986 when poinsettia growers in Florida experienced devastating outbreaks of what was at first called sweetpotato whitefly, Bemisia tabaci. Sweetpotato whitefly had been in the U.S. since 1897 but was not a serious agricultural pest. To distinguish this new, damaging form of sweet potato whitefly, from the pre-existing, less damaging strain, the old strain was referred to as strain A, and the new strain damaging poinsettia, field crops, and vegetables, was called strain B. By 1987-88, B. tabaci strain B had spread to almost every poinsettia growing region in the United States. Work on the biology, morphology, and genetics of strains A and B revealed sufficient difference that strain B was described as a new species of whitefly. Strain B was named Bemisia argentifolii, the silverleaf whitefly, because of its ability to cause squash silverleaf. The exact country of origin of B. argentifolii is unknown, but it is thought to be India or Pakistan as this area shows the greatest diversity in parasitoids of Bemisia, which may be an indication of a genus epicenter.
Pest Identification and Biology |
Pest identification. An important step in any pest management program is the accurate identification of the pest. This is particularly true for biological control because natural enemies are often specific to just one pest or group of pests e.g. whiteflies. Adults and pupae of B. tabaci strain B are readily distinguishable from those of T. vaporariorum. Bemisia tabaci strain B adults are smaller than adult T. vaporariorum, are more active, and hold their wings in a tent like fashion against the sides of the abdomen. Trialeurodes vaporariorum adults hold their wings flat over the top of the abdomen almost parallel to the leaf surface. B. tabaci strain B pupae are yellow with reddish colored eyes, oval in shape, and lack long waxy spines. Conversely, T. vaporariorum pupae are white, have an elevated straight-sided body wall with a fringe of waxy spines. Trialeurodes vaporariorum females often lay their eggs in circles or semi-circles, and as the eggs mature they turn from white to dark purple in color. Bemisia tabaci strain B females lay their eggs haphazardly over the underside of the leaf (occasionally females will lay eggs in circles). As eggs mature they turn from white to amber.
Whitefly biology. Whiteflies (Homoptera: Aleyrodidae), are small plant-feeding insects with piercing-sucking mouthparts, and both immature and adult whiteflies feed on the undersides of leaves. Adult whiteflies have the ability to both walk and fly, and females lay eggs either singly in a haphazard manner or in spirals or circles on the undersides of leaves. Whitefly eggs are ovoid and have a peg-like pedicel that is inserted into a slit made by the female’s ovipositor in the leaf surface. Alternatively, eggs may be laid directly into stomatal openings. A glue-like substance deposited at the base of the pedicel cements eggs in place. The pedicel draws water into the egg from the leaf thereby preventing desiccation before hatching.
Most whitefly species are arrhenotokous, and females are produced from fertilized eggs. Males are haploid and eclose from unfertilized eggs. The ratio of male and female whiteflies in a population changes over time and is affected by both temperature and male longevity. Males tend to live for shorter periods and populations appear female biased as a result.
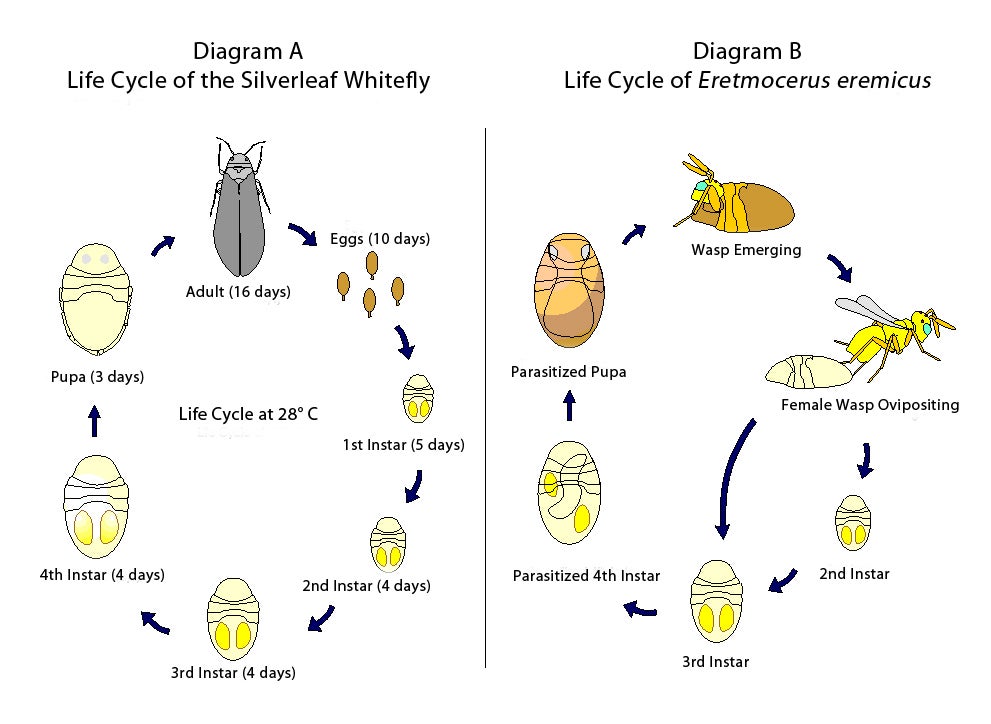
First instar nymphs which hatch from eggs are mobile, and walk a short distance before selecting sites where they settle to commence feeding. This ambulatory first instar is referred to as the crawler, and crawlers may walk for several hours and cover distances up to 30 mm before settling. After settling, crawlers insert their mouthparts into leaf tissue, and the stylet passes between host cells until the phloem is penetrated and sap extraction begins. A modified alimentary system concentrates sugars in the anterior midgut while excess fluids are diverted to the hindgut and excreted. The subsequent developmental stages (the second, third, fourth, and pupal [the last, non-feeding portion, of the fourth instar]) are sessile and do not move from the feeding site originally selected by the crawler. Diagram A summarizes the life cycle of B. tabaci Strain B.
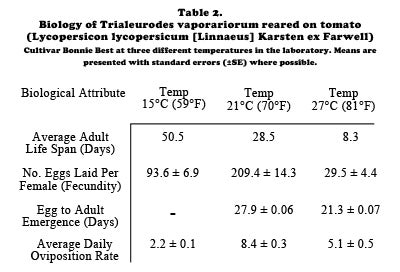
Much of what is known about whitefly biology comes from research on pest species, such as sweetpotato whitefly, Bemisia tabaci (Gennadius), greenhouse whitefly, Trialeurodes vaporariorum (Westwood), and silverleaf whitefly, Bemisia argentifolii Bellows and Perring (also referred to as the B strain or biotype B of B. tabaci) Important aspects of the developmental and reproductive biology of B. tabaci strain B and T. vaporariorum are presented in Table 1 and Table 2, respectively.
Honeydew. Whitefly waste products resulting from feeding are referred to as honeydew. These viscous liquids contain high concentrations of various sugars. Intracellular symbiotic bacteria in the whitefly gut convert the sucrose found in plant sap into a variety of sugars which are excreted. Less honeydew is produced by nymphs on well fertilized plants, as nymphs on poor quality hosts must feed more to satisfy their dietary requirements. Adult female whiteflies produce the most honeydew of any lifestage, and honeydew excretion is continuous and not restricted to certain periods of the day. Whiteflies have a unique method for disposing of honeydew. The anus of whitefly nymphs opens into a depression on the dorsal surface called the vasiform orifice. A mobile structure known as the lingula dips into honeydew as it pools in the vasiform orifice and upon release the lingula flicks the honeydew off the nymph.
Feeding Damage and Impact to Plants |
Host plants and feeding damage. Bemisia tabaci strain B and T. vaporariorum are cosmopolitan in distribution and are extremely polyphagous having been recorded from over 60 different plant families. Trialeurodes vaporariorum has been recorded as a pest from greenhouse grown Arum spp., Begonia sp., Coleus sp., Fuschia sp., Pelargonium sp., Primula sp., Verbena sp., and Euphorbia spp. (e.g. poinsettias). Bemisia tabaci strain B infests a variety of greenhouse ornamentals including poinsettias, Pelargonium sp., Impatiens sp., Gerbera sp. Hibiscus sp., and Begonia sp.. Different host plant species can significantly affect whitefly survivorship and reproductive rates For example, at 25oC, egg to adult development for B. tabaci strain B is fastest on begonia (20 days), slowest on gerbera (29 days) and intermediate on hibiscus (25 days) and poinsettia (23 days).
| Figure 2. Poinsettias |
Direct feeding damage. Whiteflies can cause direct damage to plants as a result of feeding. Bemisia tabaci strain B vectors geminiviruses that cause yellowing and leaf curling in several vegetable crops, and feeding by nymphs can induce physiological disorders such as irregular ripening in tomatoes and silverleaf in curcubits. Vein clearing of foliage and induction of white stems on poinsettia and other ornamentals can occur as a result of feeding by B. tabaci strain B. However, the ability of this whitefly to vector viral diseases affecting ornamentals is not a generally recognized problem. Trialeurodes vaporariorum vectors closteroviruses that affect cucurbitaceous species, and documented economic losses from viruses vectored by T. vaporariorum in ornamental crops are lacking. Feeding by B. tabaci strain B on poinsettias can reduce plant height, leaf number, leaf size, and dry weight yields.
Indirect feeding damage. Honeydew deposition on leaves produces a shiny, sticky sheen, and provides an ideal substrate for sooty mold growth (e.g., Cladosporium and Alternaria spp.). Declines in the aesthetic quality of ornamental plants in greenhouses because of honey dew, black sooty mold contamination, or flying adults are problems associated with high density whitefly populations. Disfigurement of plants in this manner by whiteflies is referred to as indirect damage as it has not resulted from physical damage caused by whitefly feeding and consumer acceptance for ornamental plants with indirect damage is low.
Whitefly distribution within plants and inter-specific competition. All developmental stages of T. vaporariorum and B. tabaci exhibit aggregation within and between ornamental plants in greenhouses. Whitefly lifestages are vertically stratified in a plant with respect to leaf age. Adults, eggs, and first instar nymphs are found on predominantly on young leaves, second and third instar nymphs on middle aged leaves, and fourth instars, pupae, and pupal cases on middle aged and old leaves. Consequently, population estimates can be based on whitefly counts made from leaves in these three age categories. Adult whiteflies can be monitored in greenhouses with yellow sticky cards. Placement of cards five cm above the crop canopy produce the most abundant adult catches. Adults of B. tabaci and T. vaporariorum tend to exhibit peak flight activity in the morning and afternoon, respectively.
On poinsettias, B. tabaci out competes and may displace T. vaporariorum within two generations when these species co-exist simultaneously over the temperature range 20oC to 30oC. Displacement of T. vaporariorum by B. tabaci may be related to host plant suitability, with poinsettias favoring increased oviposition and immature survivorship of B. tabaci. Aggregations of adult B. tabaci on leaves initiates avoidance behavior in adult T. vaporariorum which rarely settle in close proximity to B. tabaci.
Biological Control Approaches |
Natural enemies (predators, pathogens, and parasitoids) debilitate organisms either by prematurely killing the target (i.e., the pest) through predation, disease or parasitism, or by reducing the reproductive output or competitiveness of the pest. Biological control is the intentional use of natural enemies by humans to control pest populations. Predators, pathogens, and parasitoids are commercially available for use in greenhouses against T. vaporariorum and B. tabaci strain B infesting ornamentals.
Whitefly Predators
Predators are organisms that obtain energy as food by consuming more than one individual prey over the course of their lifetime. Often, both immature and adult stages are predatory. A notable exception are some species of adult green lacewings (e.g., Chrysoperla carnea [Stephens]), where the adults feed on plant exudates or honeydew.
Bemisia tabaci strain B is attacked by predatory species representing eight arthropod orders, including members of the families Phytoseiidae (Acari), Coccinellidae (Coleoptera), Syrphidae (Diptera), Anthocoridae, Nabidae, and Miridae (all Hemiptera), Chrysopidae and Coniopterygidae (both Neuroptera). At least four species of predators that are commercially available have been evaluated for their ability to control B. tabaci strain B on greenhouse grown crops: Delphastus pusillus LeConte, Macrolophus caliginosus Wagner, C. carnea, and Chrysoperla rufilabris (Burmeister).
| Figure 3. Delphastus pusillus adult |
Delphastus pusillus (Coleoptera: Coccinellidae). Delphastus pusillus is often associated with high density populations of whiteflies and feeds readily on B. tabaci strain B. Adult D. pusillus are small, shiny black beetles 1.3-1.4mm in length, and larvae and pupae are pale yellow. Beetles pupate in groups on the undersides of lower leaves or in crevices. Adults and immature beetles feed by piercing the whitefly integument and extracting the contents resulting in a flattened, empty whitefly cuticle. Adults and all larval stages will feed on whitefly eggs, nymphs, and adults. In artificial arenas, female fecundity is greatest on a diet of eggs, but in cage studies with small poinsettia plants, B. tabaci strain B nymphs were preferred over eggs.
Leaf hairs can affect life-history traits and searching ability of D. pusillus. For example, leaf trichome densities on poinsettia vary depending on cultivar. The cultivar "Annette Hegg Brilliant Diamond" has trichome densities 15% lower than "Lilo". On the least hairy variety, prey consumption, and oviposition rates by D. pusillus are significantly higher in comparison to more hirsute varieties.
Delphastus pusillus larvae and adults avoid feeding on whiteflies with late stage developing parasitoids (Hymenoptera: Aphelinidae) (i.e., parasitoid immatures >7 days of age) (e.g., Encarsia transvena (Timberlake), Encarsia luteola Howard, and Eretmocerus sp. nr californicus Howard) and preferentially attack unparasitized prey. Biological control with D. pusillus is most effective when this predator is combined with one or more species of aphelinid parasitoid. For example, D. pusillus combined with E. luteola at a weekly release rate of one beetle and one parasitoid per plant can control B. tabaci strain B to levels attained with insecticides.
Macrolophus caliginosus (Hemiptera: Miridae). This predatory bug is polyphagous and feeds on spider mites (Acari: Tetranychidae), aphids (Homoptera: Aphididae), whitefly nymphs and eggs, leaf miners (Diptera: Agromyzidae) and lepidopterous pests. Macrolophus caliginosus is a partial predator and needs to supplement its diet by feeding on plant material to complete its lifecycle, and damage to plants can occur in some ornamental crops as a result of phytophagy when there is a lack of prey. Phytophagy, however, allows M. caliginosus to establish early in greenhouses when prey densities are low and to persist if pest densities decline. Three releases of this predator at a rate of 0.5-1 per m2 at two week intervals in combination with weekly releases of Encarsia formosa Gahan (Hymenoptera: Aphelinidae) has provided successful biological control of low and high density populations of T. vaporariorum in tomatoes. Release of two M. caliginosus per m2 is recommended if whitefly densities are close to economically damaging levels.
| Figure 4. A green lacewing (Chrysoperla carnea) adult. |
Chrysoperla spp. (Neuroptera: Chrysopidae). Ten species of lacewings have been reported to feed on whitefly nymphs, including the commercially available species C. carnea and C. rufilabris. The three larval stages of C. carnea will feed on B. tabaci and T. vaporariorum eggs, nymphs, and pupae. However, C. carnea is unable to develop to adulthood on a diet consisting only of T. vaporariorum. Similarly, C. rufilabris larvae will feed on all immature stages of B. tabaci, but they are unable to reach adulthood on this diet alone. Also, larval developmental times on whiteflies are substantially longer than those of lacewing larvae reared on lepidopteran hosts or a diet composed of several types of food. In the absence of prey, C. rufilabris can sustain itself on nutrients extracted from leaves for 6-7 days.
Despite being an unsuitable host, B. tabaci infesting greenhouse grown hibiscus plants (Hibiscus rosa-sinensis Linnaeus) can be controlled to commercially acceptable levels with inundative releases of C. rufilabris. Two releases two weeks apart of either 25 or 50 larvae per plant consistently produced marketable plants. Releases of five C. rufilabris larvae per plant produced inconsistent levels of control. Releases of C. carnea larvae in combination with E. formosa have suppressed T. vaporariorum on marigolds (Tagetes erecta L.).
Whitefly Pathogens
Insect pathogens are disease-causing organisms which either kill or debilitate the host and include bacteria, viruses, protozoa, and fungi. Owing to the feeding mechanisms of whiteflies, only pathogenic organisms with the ability to penetrate the cuticle (e.g., fungi) have potential as microbial control agents. The most commonly observed fungal pathogens of whiteflies are Paecilomyces fumosoroseus (Wize) Brown and Smith, Aschersonia aleyrodis Webber, Verticillium lecanii (Zimmerman) Viegas, and Beauveria bassiana (Balsamo) Vuillemin. Of these, V. lecanii, B. bassiana, and P. fumosoroseus are commercially available.
Verticillisum lecanii (Deuteromycotina: Hyphomycetes). This pathogen attacks aphids, scales, thrips, whiteflies, and other fungi: if also has the ability to act as a saprophyte and feed upon non-living organic substrates. Epidemics of V. lecanii can occur naturally in greenhouses and the inoculum for initial infection is probably present in some potting media. Verticillium lecanii develops at temperatures ranging from 15 to 25oC and relative humidities of 85-90%, which must exist for 10-12 hours each day. Spore solutions of V. lecanii sprayed onto poinsettia leaves with eggs, first, second, and third instar nymphs of B. tabaci or T. vaporariorum caused mortality ranging from 89 to 96% for B. tabaci and 79 to 96% for T. vaporariorum. Eggs of both whitefly species exhibited low rates of infection (<1%), and adult whiteflies are relatively immune to infection also. Verticillium lecanii has been used to control T. vaporariorum on greenhouse grown cucumbers and B. tabaci on melons. Certain fungicides are harmful to V. lecanii and should not be used simultaneously with this pathogen.
Beauveria bassiana is not a natural regulator of whitefly populations, and is only occasionally found infesting whiteflies in agroecosystems. In comparison to other fungal pathogens, little work has been done to evaluate the efficacy of this natural enemy against whiteflies. Commercially available strains of B. bassiana exhibit high levels of pathogenicity in laboratory studies and B. tabaci nymphs and adults are susceptible to infection. Temperature and humidity levels affect B. bassiana pathogenicity and maximum mortality is inflicted at low temperatures (< 20oC) and high humidity (> 96%) when these conditions persist for at least 24 hours. Whitefly nymphs infected with B. bassiana display a pronounced red pigmentation. Oosporein, a dibenzquinone, is responsible for the red color and its antibiotic properties aid the infection process.
The efficacy of B. bassiana can be enhanced if this pathogen is used in combination with insecticides which stress the target pest. Sublethal doses of imidacloprid (a chloronicotinyl insecticide commonly used against whiteflies) in combination with B. bassiana have a synergistic effect and cause higher levels of mortality than using either compound alone for weevil control. Imidacloprid applications increase the rate and amount of B. bassiana conidial germination on the insect cuticle. Similarly, combining B. bassiana with diflubenzuron (an insect growth regulator) has an additive effect for grasshopper control as this IGR enhances the pathogen’s infection process. Similar data investigating the efficacy of combining B. bassiana with insecticides for whitefly control are currently unavailable and research in this area is warranted. Commercially available fungicides (e.g., maneb, mancozeb, and thiophanate-methyl) inhibit mycelial growth and sporulation by B. bassiana and careful consideration should be given when using these chemicals with entomopathogenic fungi.
Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes). Paecilomyces fumosoroseus has a broad host range and have been recorded from over 40 different insect species. Hosts for this fungus include whiteflies, mealybugs, beetles, caterpillars, and flies. Natural epizootics of P. fumosoroseus infesting B. tabaci in greenhouses and shade-houses have been recorded.
The range of whitefly lifestages infected by P. fumosoroseus is broader than most entomopathogenic fungi and it is able to attack eggs, nymphs, pupae, and adults of B. tabaci and T. vaporariorum. Conditions which favor infection of whiteflies by sporulating P. fumosoroseus conidia are when temperatures range from 22oC to 33oC and relative humidities are 68% to 100%. The susceptibility of B. tabaci to infection by P. fumosoroseus is not effected by the host plant being used by the whitefly. In greenhouses, second instar B. tabaci nymphs were equally susceptible to P. fumosoroseus infection on cucumbers (Cucumis sativus L.), cabbage (Brassica sativa L.) and three cultivars of tomato under optimal environmental conditions.
Foliar applications of P. fumosoroseus conidia have been successfully combined with E. formosa in commercial greenhouses for control of T. vaporariorum on tomatoes. Trialeurodes vaporariorum nymphs parasitized by E. formosa and have turned black are immune from infection by P. fumosoroseus making simultaneous use of these two natural enemies compatible. Compatibility of P. fumosoroseus with Eretmocerus sp. and D. pusillus has been observed. Paecilomyces fumosoroseus has been used as the sole control strategy for B. tabaci, on greenhouse grown poinsettias and hibiscus (Hibiscus rosa-sinensis L.). To produce plants of the same quality as those protected by P. fumosoroseus, poinsettias required 18 insecticide treatments for B. tabaci control while hibiscus plants were treated 21 times. Laboratory tests indicate that P. fumosoroseus has a high tolerance to a broad range of fungicides which are frequently used to protect ornamental plants in greenhouses.
Aschersonia aleyrodis (Deuteromycotina: Coelomycetes). Members of the genus Aschersonia are highly specific to whiteflies and A. aleyrodis infects and kills nymphs and pupae, but not eggs of T. vaporariorum and B. tabaci strain B nymphs on poinsettias and cucumbers. Trials in vegetable crops indicate that ultra low volume sprays of conidial suspensions can cause 75% mortality to T. vaporariorum under varying climatic conditions. Spores can germinate at temperatures ranging from 15 to 30oC if relative humidities are between 98 and 100%. Aschersonia aleyrodis can be combined with the parasitoid E. formosa for T. vaporariorum control. Parasitized whitefly nymphs in which immature parasitoids are greater than four days old are immune to infection by A. aleyrodis. The developing wasp larva induces a physical or physiological changes in the host that reduces its susceptibility to fungal infection. Similarly, E. formosa is able to discriminate and reject whitefly hosts that have been infected with A. aleyrodis for at least seven days. If hosts are parasitized before this seven day period, A. aleyrodis inhibits parasitoid development. Aschersonia aleyrodis and E. formosa, appear to be highly compatible natural enemies for combined used against T. vaporariorum in greenhouses.
Whitefly Parasitoids
Parasitoids are distinguished by the fact that the immature stages develop at the expense of a single individual which is referred to as the host. Parasitoids differ from parasites in that they kill their host upon completing development. Whitefly parasitoids belong to just three hymenopterous families Platygasteridae (e.g., Amitus spp.), Aphelinidae (e.g., Eretmocerus and Encarsia spp.), and the Eulophidae (e.g., Euderomphale spp.). The best studied of these whitefly parasitoids are E. formosa and Eretmocerus eremicus Rose and Zolnerowich, both of which are commercially available.
Encarsia formosa (Hymenoptera: Aphelinidae). Encarsia formosa is used globally for the control of T. vaporariorum on greenhouse-grown vegetable crops and to a lesser extent ornamental crops. This parasitoid is a solitary, thelytokous (unfertilized eggs produce female offspring), endoparasitoid (parasitoid develops inside the body of the host). Females preferentially deposit single eggs in third and fourth instar whitefly nymphs. Adult wasps obtain energy and nutrients by piercing the integument of nymphs with the ovipositor and feeding on hemolymph that seeps from the wound. Killing hosts for adult nutritional purposes is termed host feeding. On some plants the searching efficacy of E. formosa is reduced by high trichome densities and this has made whitefly control on some crops like gerbera more difficult to achieve.
| Figure 5. Encarsia formosa, Beltsville strain |
Bemisia tabaci strain B on poinsettia is an unsuitable host for E. formosa, because parasitoid development is slower, more immature parasitoids die, and adults are less fecund in comparison to wasps reared in T. vaporariorum on the same host plant. In small experimental greenhouses, E. formosa and a Bemisia-adapted strain of E. formosa (commonly referred to as the "Beltsville strain") appeared to be promising biological control agents of B. tabaci strain B. However, in commercial greenhouses weekly releases of three or more E. formosa per week failed to control pure populations of B. tabaci strain B on poinsettia stock plants or colored poinsettias. Consequently, use of this parasitoid for control of B. tabaci strain B is not recommended. Improved control of B. tabaci, strain B may be achieved when T. vaporariorum, (the preferred host) is present in the crop. Aspects of the biology of E. formosa reared on either B. tabaci strain B or T. vaporariorum are presented in Table 3.
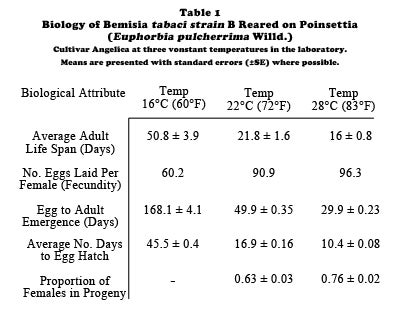
Eretmocerus eremicus (Hymenoptera: Aphelinidae). This parasitoid was formerly known as Eretmocerus sp. nr. californicus and it is a primary, bi-parental, solitary ecto-endoparasitoid (part of the lifecycle is spent feeding on the outside of the host before parasitoid development is completed inside the host) of whiteflies with a 1:1 sex ratio in mass culture. Females are easily distinguished from males by having a pronounced club on the end of the antenna which males lack. Females oviposit under suitable hosts, and parasitoid larvae, after hatching, penetrate the ventral surface of the host and develop as endoparasitoids inside the host. Eretmocerus eremicus host feeds by inserting its ovipositor in the vasiform orifice of the host. Weekly releases (three female wasps per plant per week) of E. eremicus have proven to be more effective than the same release rate of E. formosa for controlling B. tabaci strain B on poinsettias. In commercial greenhouses, visual inspections of experimental plants with artificial whitefly patches indicated that E. eremicus finds whitefly patches faster and consistently kills more nymphs after discovery than E. formosa.
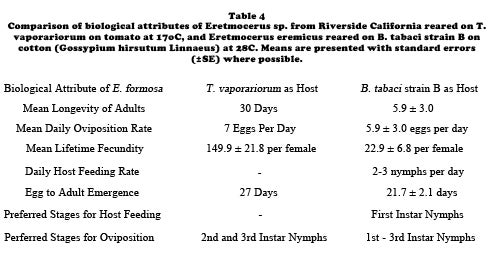
Biological control of B. tabaci strain B on poinsettias with E. eremicus may be improved by varying the weekly parasitoid release rate to concentrate parasitoid attack against whiteflies when cuttings are small and there are few leaves to search. With a variable release strategy, six female parasitoids are released per plant per week for the first half of the growing season. This rate is then reduced to one female parasitoid per plant per week for the remainder of the growing season. The assumption is low level releases coupled with parasitoid reproduction will maintain whitefly densities at non-damaging levels until harvest because of high levels of mortality inflicted on whiteflies by parasitoids during the high release rate phase. With this variable release rate strategy, the overall release rate is still three female wasps per plant per week. Growers have successfully used E. eremicus to control pure populations of either B. tabaci strain B or T. vaporariorum in commercial poinsettia ranges. Aspects of the biology of Eretmocerus spp. reared on either B. tabaci strain B or T. vaporariorum are presented in Table 4. The life cycle of E. eremicus is shown in Diagram B.
Economics of Whitefly Biological Control and Future Prospects
Presently, control of whiteflies with natural enemies alone is more expensive than using insecticides. The combined use of E. luteola and D. pusillus provided acceptable control of whiteflies in comparison to insecticide applications but was five times more expensive. Similarly, the use of E. eremicus alone at rate of three females per week per plant is 27-44 times more expensive than using insecticides. However, it is possible to totally eliminate the use of insecticide applications through the use of E. eremicus for whitefly control on poinsettia stock plants and colored plants grown for sale at Christmas. Furthermore, when releases of natural enemies are used in combination with insecticides, spray applications can be reduced by 75% and control costs can become comparable to those of foliage-applied insecticides.
To be economically feasible, either lower release rates of E. eremicus need to be used or the cost of the parasitoid needs to be reduced. In 1994, E. eremicus (incorrectly marketed as Eretmocerus californicus Howard) was offered commercially for the first time at a cost of $76 per 1000 parasitized nymphs. In 1995, the price dropped to $22, and in 1998 the cost fell further to $8.30 per 1000 parasitized nymphs, a decrease of 89% from the original 1994 retail price. Further price reduction for E. eremicus can be expected to continue if parasitoid production efficiency continues to improve and demand increases.
Lower release rates of E. eremicus (as low as one female parasitoid per plant per week) can provide adequate whitefly control in commercial poinsettia crops if supplemented with limited use of compatible insect growth regulators (IGR’s). Results indicate that an E. eremicus-IGR combination is more effective than use of either an IGR or E. eremicus alone, and IGR’s compatible with E. eremicus have been identified from laboratory studies. Resistance development by B. tabaci strain B to imidacloprid, the main pesticide used for control of this pest on poinsettias is expected as it has been detected in Europe on vegetable crops. Resistant B. tabaci strain B populations are predicted to become widespread within a short period given the extensive exchange of poinsettia cuttings and plants nationally and internationally. Incorporation of E. eremicus into an IPM program would diversify whitefly control options, reduce reliance on insecticides, enhance cost effectiveness of biological control, and provide a more sustainable whitefly control program for poinsettia production.
Initiating a Biological Control Program for Whiteflies |
When natural enemies are missing from an agricultural setting (e.g., greenhouses immediately after planting), or are too scarce to provide control, their numbers can be increased by making releases of agents purchased from insectaries. This approach is termed augmentative biological control and either inoculative or inundative approaches are used. Inoculative releases are those in which small numbers of natural enemies are introduced into the greenhouse early in the crop production cycle and subsequent control occurs as natural enemies reproduce and feed on the pest. This approach has been used to control T. vaporariorum on greenhouse grown tomatoes with E. formosa.
Inundative or mass releases are used when natural enemy reproduction is expected to be insufficient to suppress pest population growth, and control is achieved by released natural enemies and to a limited extent their offspring. Inundative biological control is the approach used for rapid pest suppression on ornamentals because of low tolerances for aesthetic damage and arthropod contamination of the saleable product. Preventative inundative natural enemy releases are started early in the growing season when pest densities are well below those that cause economic damage, and pest population growth is prevented from reaching damaging levels. Inundative natural enemy releases can not be used in "curative" manner to reduce pest populations to non-damaging levels once they have reached economically injurious densities. The steps recommended for initiating a preventative inundative biological control program with E. eremicus for B. tabaci strain B on poinsettias are outlined below.
If whitefly populations are either T. vaporariorum, B. tabaci strain B, or a mixture of both whiteflies, the use of E. eremicus is recommended as a biological control agent. Success can be further enhanced by developing an effective scouting program and using either trained staff or hiring a professional IPM scout to identify whitefly species and monitor pest and parasitoid population growth.
Prelease crop inspection. Before releases of E. eremicus are made, cuttings need to be inspected for the presence of whitefly nymphs and adults. To determine initial infestation levels, whitefly nymphs on every leaf of 50 randomly selected cuttings should recorded. If mean densities of whitefly nymphs are < 2 per cutting initial infestation levels are low enough to commence weekly parasitoid releases. If nymph densities are > 2 per cutting, alternative control measures should be followed. These may include either the use of insecticides which leave persistent residues. Long-lived residues may prevent the use of natural enemies for several weeks thereby making a biological control program impractical to implement. Alternatively, the use of chemicals compatible with parasitoids to reduce densities of whitefly nymphs to levels < 2 per leaf may be used.
Ordering and deploying natural enemies. A weekly release rate of three female E. eremicus per plant per week for the duration of the growing period can be used. At this release rate, E. eremicus provides effective control by itself. However, the total cost of purchasing parasitoids can be reduced by employing a lower weekly release rate (i.e., one female parasitoid per plant per week) in combination with two applications of an effective insect growth regulator (IGR) (e.g., buprofezin) made one week apart at the mid-point of the growing season. Parasitoid releases are then resumed following IGR applications.
After shipment E. eremicus emergence from parasitized T. vaporariorum nymphs placed in greenhouses averages 60%. When an order is placed, commercial insectaries may over supply parasitoids to compensate for reduced parasitoid emergence. For example, Koppert Biological Systems supplies a 142% of the number of E. eremicus requested because they estimate that emergence will be 70%.
Eretmocerus eremicus are shipped as parasitized T. vaporariorum nymphs and may be either packaged in sawdust or glued to release cards that are placed in the greenhouse. Material in sawdust can be placed in release cups and distributed at a rate of one cup per 18m2 of greenhouse. Eretmocerus eremicus is highly vagile and disperses readily within greenhouses, thereby making large numbers of release sites unnecessary. Samples of recently purchased material should be examined to ensure that parasitoids are arriving in good condition and are emerging successfully. This can be done by placing 50-100 parasitized whitefly nymphs in a clear glass jar and counting the number of parasitoids that have emerged after 5-7 days. If a high number of yellow parasitoids are seen, the shipment can be assumed to have arrived in good condition. If few parasitoids are observed, inform the supplier that shipment quality was poor and negotiate for a replacement order or change supplier.
Monitoring whitefly populations and determining parasitoid efficacy. Once releases of E. eremicus begin, plants should be inspected weekly to measure the effectiveness of parasitoid releases. Records of the numbers of live whitefly nymphs, pupae, and adults should be kept. These records will indicate whether whitefly populations are increasing, decreasing, or remaining stable. Should whitefly numbers begin to increase to levels that are unacceptable, use of an insecticide that is compatible with E. eremicus (e.g., use of insect growth regulators like buprofezin) should be used to reduce whitefly numbers and parasitoid releases should be re-initiated. At time of harvest, densities of live nymphs and pupae should average 0.55-1.5 per leaf, and this level of control has been achieved with weekly releases of E. eremicus. Final nymph densities within this range are acceptable to consumers, and similar to those attained with insecticides.
Additional Resources |
For further information on biological control and research on whiteflies attacking ornamental crops contact:
- Mark Hoddle, Dept. of Entomology, University of California, Riverside, CA 92521, phone no. (909) 787-4714, fax no. (909) 787-3086, e-mail mark.hoddle@ucr.edu
- Roy Van Driesche, Dept. of Entomology, University of Massachusetts, Amherst, MA 01003, phone no. (413) 545-1061, fax no. (413) 545 2115, e-mail vandries@cns.umass.edu
- John Sanderson Dept. of Entomology, Cornell University, Ithaca, NY 14853, phone no. (607) 255 5419, fax no. (607) 255 1720, e-mail jps3@cornell.edu
- Tina Smith UMass Extension, Univeristy of Massachusetts, Amherst, MA 01003, phone no. (413) 545-5306, e-mail tsmith@umext.umass.edu
- Julie Newman, Ventura County Farm Advisor, 669 County Square Drive #100, CA 93003, phone no. (805) 645-1459, fax no. (805) 645-1474, e-mail jpnewman@ucdavis.edu