Assessing the use of cover crops for sustainable pest control in California vineyards (funded by Western SARE)
Prepared by Nic Irvin, Biological Control Research Specialist, with contributions by Allison Bistline, Lab Assistant
nic.irvin@ucr.edu
Introduction |
Work completed and recorded on this webpage is the first significant set of studies that have investigated the strengths and limitations of cover cropping under the unique growing conditions representative of grape producing areas of Southern California. This four-year project was funded by Western Region of Sustainable Agriculture Research and Education (Western SARE) and began in June 2007. The project investigated the use of buckwheat (Fagopyrum esculentum Moench) and cahaba vetch (Vicia sativa L.) for management of arthropod grape pests in California and included a large-scale field trial located at Bella Vista Vineyard in Temecula.
|
Figure 1. Buckwheat (Fagopyrum esculentum) in a cover crop plot. |
The Californian wine industry promotes sustainable practices through the Code of Sustainable Winegrowing Workbook (CSWW) (Dlott et al., 2002), however, sustainability is severely affected by the glassy-winged sharpshooter problem in Temecula which triggers growers to apply prophylactic Admire applications to prevent spread of Pierce’s Disease. This costs approximately $225/acre (Ben Drake, pers. comm.). An alternative option for pest control is required to help reduce pesticide reliance and promote sustainable pest management. Research shows that cover crops are beneficial for enhancing natural enemies of vineyard pests, and controlling spider mite and leafhopper populations in vineyards (Altieri & Schmidt 1985; Settle et al., 1986; Hanna et al., 1996; Costello & Daane, 1998a,b; Klonsky et al., 1998; Nagarkatti et al., 2003). Maintaining cover crops in vineyards can enhance natural enemy populations by providing a habitat, shelter, nectar and alternative food for predators and parasitoids. However, Southern Californian’s semi-arid conditions limit the use of year-round cover cropping and cause weeds between vine rows to die over the spring/summer period making the vineyard an unfavorable habitat for natural enemies at this critical time for pest control. Our Western SARE project proposed that maintaining a ‘nectar cover crop’ throughout the spring and summer through additional irrigation may further enhance natural enemy populations, supply natural enemies with nectar to increase survival and fecundity, resulting in pest numbers being consistently maintained below economic thresholds.
There were 7 objectives this study sought to determine:
|
It is important to select cover crops that will benefit natural enemies that can reduce pest populations, while simultaneously having no detrimental effects on vine growth, yield or grape quality. Buckwheat (Figure 1) shows promising potential as a cover crop in vineyards as it germinates easily, has a short sowing-flowering time, and its seed is inexpensive and readily available.
Previous studies show that buckwheat increased longevity and fecundity of parasitoids in the laboratory that attack sharpshooters, a key pest of grapes (Irvin & Hoddle, 2006), and led to lower abundance and higher parasitism of leafhoppers in vineyards (Nicholls et al., 2002, English-Loeb et al., 2003). Cahaba vetch (Figure 2) suppresses populations of damaging nematode species in California vineyards (McKendry, 1992), and this plant is suggested in Code of Sustainable Winegrape Workbook (CSWW) (Dlott et al., 2002) as a cover crop option to improve soil nutrition, fertility and structure, and to reduce erosion and dust. The Western SARE project aimed to investigate whether the extrafloral nectaries of cahaba vetch can be utilized as food by parasitoids of grape pests.
Parasitoid Survival and Fecundity on Nectar Resources |
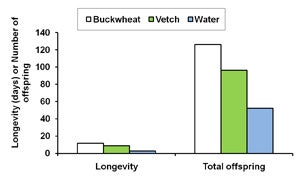
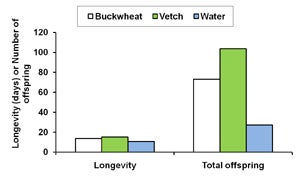
| A. |
| B. Diagram 1. The effect of buckwheat, vetch, and water as food sources on the survival and fecundity of A. Gonatocerus ashmeadi (GWSS parasitoid) and B. Anagyrus pseudococci (vine mealybug parasitoid) in the laboratory. |
The first step of this trial was to examine whether buckwheat flowers and cahaba vetch extrafloral nectaries increase longevityand fecundity of natural enemies G. ashmeadi (GWSS parasitoid) and Anagyrus pseudococci (vine mealybug parasitoid). Results from laboratory trials showed that female A. pseudococci provided with vetch plants survived 4 and 5 days longer, respectively, than on water alone. The total number of offspring was nearly four times higher for females provided with either one of these nectar sources than for females with water only (Diagram 1B). Similarly, providing G. ashmeadi females with buckwheat (Figure 3) and vetch plants (Figure 4) significantly increased longevity compared with water, with buckwheat-fed females living nearly twice as long as vetch-fed females. Numbers of offspring produced by females on buckwheat or vetch plants was also nearly 150% higher compared with females provided only water (Diagram 1A). These results suggest that vetch and buckwheat are suitable food sources for both A. pseudococci and G. ashmeadi and may show potential for enhancing longevity and fecundity of these parasitoids if grown as a cover crop. This increase in parasitoid fitness, brought about by more ready access to nectar resources, may then enhance biological control of mealybugs and GWSS through an increase in parasitism rates.
Cover Crop Phenology |
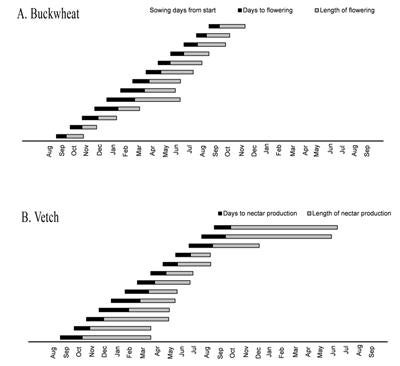
Cover crop phenology trials were conducted beginning in June 2007 at UCR Agricultural Operations (AgOps). In the middle of each month over the course of one year, five buckwheat (Figure 5) and five vetch (Figure 6) plots were sown, and plots were examined every six weeks to measure plant height, sowing to flowering times, and length of flowering period. Mean six-week height varied for both species, with shorter plants having been sown in the winter months. This information may be useful for growers who are selecting cover crops for crops that require an open canopy for prevention of moisture-loving diseases.
|
Diagram 1. Growers guides for strategizing (a) buckwheat and (b) cahaba vetch plantings to maximize nectar production at critical times for pest control. |
The number of days required from sowing to flowering for buckwheat was shortest between July and August. From April through September it took buckwheat less than one month from sowing to produce nectar-producing flowers. This is information is important for growers intending to synchronize buckwheat nectar production to the phenology of natural enemies of key pests. Vetch took 0.6-28 days longer each month to start producing nectar compared with buckwheat. Buckwheat, therefore, may be a better cover crop for growers seeking a quick-growing plant that will provide a supplemental nutrition source for natural enemies, particularly in the summer months when buckwheat produces nectar nearly one month faster than vetch. Conversely, once extrafloral nectaries developed on vetch plants, they produced nectar for up to 5 months longer than buckwheat flowers. This trade-off between speed of floral production and longevity of floral production suggests that mixed-species sowings may be useful to take advantage of both quick-flowering species as well as species with long flowering periods.
Information on days to nectar production and the length of nectar producing period for buckwheat and vetch were used to construct guides to potentially assist growers with cover crop sowing decisions. Diagram 1 portrays guides growers could use for strategizing buckwheat and cahaba vetch plantings, respectively, to maximize nectar production at critical times for pest control. Growers could select a month along the x-axis where they require increased biological control for a particular pest problem, then the duration of flowering/nectar production information could be used to determine which month the grower would need to sow buckwheat or vetch to maximize nectar production for natural enemies when the pest occurrs. For example, for grape growers in southern California, the grower guides indicated that sowing buckwheat in mid-March would supply nectar early in spring to increase fecundity and longevity of parasitoids and help replenish populations of natural enemies after over-wintering. The window of vulnerability to PD in southern California is June through August (CDFA 2010). An additional sowing of buckwheat in mid-May would supply nectar until August.
Natural Enemy Enhancement and Pest Population Suppression |
Experimental setup and design. In 2008 and 2009, a cover crop field study was set up at the vineyards of Bella Vista winery in Temecula, CA aimed at investigating the effect of buckwheat and vetch cover crops on natural enemy and pest populations. A total of six control plots (Figure 7) and seven cover crop plots (Figure 8) were chosen randomly from four blocks of Cabernet Sauvignon grapes around the vineyard to total thirteen plots for the trial. In 2008, vetch was randomly allocated to be sown on either the north or south side of a cover crop plot and seeds were sown in mid-February. In early May, buckwheat was sown on the opposite side to vetch in each cover crop plot. Vetch plants failed to establish, so the original vetch rows were cultivated in early June and re-sown with buckwheat seeds.
Vetch proved to be a very poor competitor with common agricultural weeds, struggled to germinate and survive the warm spring conditions in Southern California, and was prone to high infestations of pests (Figure 9) that severely impeded growth of the plants. Additionally, for the 2009 trial the only supplier of cahaba vetch seeds in the US lost their entire crop due to mildew fungal disease, so vetch was not included. Buckwheat seed was re-sown in cover crop plots several times throughout the trial and watered by sprinklers attached to grape irrigation lines approximately once per week. Irrigation was also supplemented two to three times per week with water applied via a water sprayer mounted on an ATV (Figure 10). The remaining vineyard rows, including the six control plots, were maintained under general vineyard practices consisting of cultivation between rows to remove unwanted weeds.
Despite the constant maintenance of these plots, there were numerous problems associated with establishing and maintaining the cover crops during these summer trials (Figure 11). Only half of the allocated buckwheat plots were established in 2008, and all plots failed to establish in 2009. In July 2009, all field components of the trial were ceased because of this poor establishment rate.
Insects were monitored using weekly transparent sticky traps and bi-weekly funnel beat samples (Figure 12), conducted from May through August, and by sweep net samples of cover crop and grapevine foliage and leafhopper visual counts on grapevine foliage, conducted from June through August.
For the 2008 field season, cover crop plots that established buckwheat were categorized as “buckwheat treatment plots” while allocated cover crop plots that failed to establish buckwheat were designated “irrigation treatment plots”. The irrigated treatment helped determine the effect of irrigation alone on insect fauna, grape yield, fruit quality, and vine vigor. Consequently, the three treatments for the study were (1) buckwheat cover crop with irrigation; (2) irrigation with no buckwheat cover crop; and (3) cultivated control with no buckwheat cover crop and no irrigation.
Results
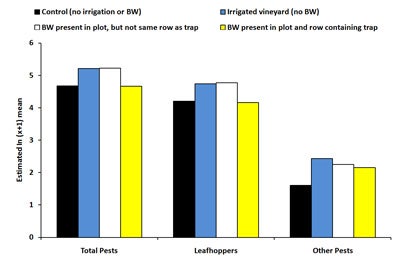
Sticky traps. There was no significant effect of treatment on the total number of pests captured on sticky traps at the beginning of the trial (June 10-August 5, 2008). For the last time period (August 12-19, 2008), total pest counts were significantly higher (72% higher) in irrigated plots compared to control plots (Diagram 3). This may be due to irrigation increasing vine vigor, making the vines more attractive to leafhoppers. Vines located in the irrigated plots and irrigated buckwheat plots had canes that were nearly 70% heavier compared to control plots, thereby illustrating increased vine vigor. The number of total pests on sticky cards was equivalent between controls and plots with buckwheat present in the same row as the trap. Numbers of pestiferous leafhoppers captured on sticky traps showed similar trends to total pest numbers, and treatments had no significant effect on the numbers of thrips collected on sticky traps across all dates.
| Diagram 3. Estimated mean number of pestiferous insects counted on sticky traps deployed in four treatments in a vineyard during August 12th, 2008 and August 26th, 2008 (BW = buckwheat). |
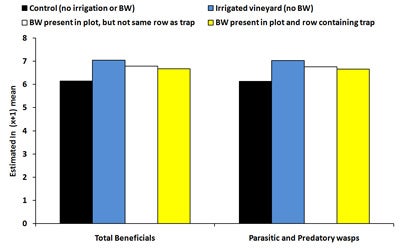
| Diagram 4. Estimated mean number of beneficial insects and parasitic and predatory wasps counted on sticky traps deployed in four treatments in a vineyard during July 29th, 2008 and August 12th, 2008 (BW = buckwheat). |
There was no significant effect of treatment on the total number of beneficial insects captured on sticky traps between mid-June and mid-July, 2008. For the last two time periods, the number of beneficials was significantly higher (146-183% higher) in irrigated plots compared to controls (Diagram 4). Between August 12-19, the number of beneficial insects was 114% higher in plots with buckwheat near the trap (but not in the same row as the trap) compared to control plots. The number of parasitic and predatory wasps captured on sticky traps showed similar trends to the total numbers of beneficial insects. Numbers of predatory thrips were significantly higher (162-186% higher) in plots containing buckwheat near the trap (but not in the same row as the trap). Overall, the number of beneficial insects was equivalent between controls and plots with buckwheat present in the same row as the sticky trap.
In summary, results from the sticky trap data indicated that irrigated plots were attractive to leafhoppers and other pests and resulted in higher pest numbers compared to controls due to increased vine vigor. However, if irrigation was coupled with presence of a buckwheat cover crop, pest numbers were similar to controls. The combined effect of buckwheat and irrigation may have been partially due to increased levels of predatory thrips in the middle of the trial, however, buckwheat failed to increase populations of parasitic and predatory wasps and other beneficial insects since numbers were equivalent between irrigated plots and plots containing irrigated buckwheat. The combined effect of buckwheat and irrigation (which cancelled the negative effects of the irrigation on pest populations) may have been attributable to buckwheat plants supplying pollen and nectar to beneficial insects and subsequently increasing longevity and fecundity, leading to higher parasitism, predation and pest control. Our laboratory studies demonstrated the positive effect of buckwheat on longevity of beneficial insects and parasitism of grape pests. Alternatively, only four replicated buckwheat plots could be established for this study which may have increased difficultly of detecting significant differences between pest numbers in buckwheat plots and controls.
Funnel beat samples. Insects collected using the funnel beat method showed a significant increase (up to nearly 9,000% of early season numbers) from June 17 through July 9, 2008. The treatment and interaction between time and treatment tests were not significantly different in any of the eight insect group models.
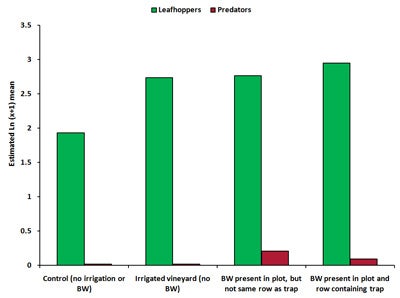
Visual counts. For visual count sampling, time had a significant effect on all insect counts, with count numbers peaking in August 2008. The maximum number of lacewing eggs occurred during July and August 2008, and occurred predominantly on the south side of the row. South sides of the vines generally receive higher sunshine hours and less protection from winds. Time and treatment had a significant effect for leafhopper and predator counts, but not for numbers of lacewing eggs. In August, numbers of leafhoppers was significantly higher (41-53% higher) in plots where buckwheat was present in the same row as the trap and in irrigated plots compared with controls. There was significantly higher numbers of predators in plots containing buckwheat near the trap compared with controls (Diagram 5).
| Diagram 5. Estimated mean number of leafhoppers and predators counted during visual inspections of grape leaves in four treatments in a vineyard during August 2008 (BW = buckwheat). |
Sweep net samples. The main objective of collecting sweep net samples from flowering buckwheat cover crop in comparison to grapevine foliage was to determine the possible risk buckwheat might pose to being a source of X. fastidiosa in the vineyard, especially since laboratory trials have shown it is possible for GWSS to transmit X. fastidiosa between buckwheat and grapevines. In all of the buckwheat cover crop plots across all sampling dates (total of 16 samples), there was only one GWSS collected, compared with over 50 GWSS collected from the grapevine foliage of control plots (total of 22 samples). These results indicate that while it is possible for GWSS to feed on buckwheat cover crops and transmit X. fastidiosa from infected buckwheat plants to grapevines, it may be unlikely that a buckwheat cover crop would act as a significant reservoir of X. fastidiosa in the vineyard since GWSS prefer feeding on grape foliage and high populations would not be found in buckwheat cover crops. Sweep net samples of buckwheat cover crop also showed a significant number of green stink bugs (which feed on developing seeds of plants) and ants. The presence of ants in the vineyard may disrupt biological control of scales, mealybugs, and aphids.
Grape Yield and Quality |
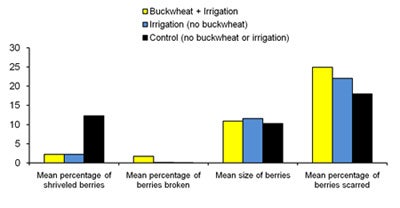
In September 2008, the number of grape clusters on a 3m section of grapevine in the center of each plot was counted, and 10 clusters were harvested from each section for laboratory analysis (Figure 13). Preliminary results show that the mean weight per cluster was 26-39% higher for those harvested from buckwheat plots and irrigation treatments when compared with controls. There was no difference in the number of clusters or number of berries per cluster between treatments. Mean Brix content (sugar content in the berry juice) was 2.5-2.9 degrees Brix higher in control plots compared with buckwheat and irrigated plots, which was probably attributable to the extra irrigation diluting the sugars in the berries or causing excess vine vigor, the latter thereby decreasing the amount of sunlight reaching the berries.
| Diagram 6. Grape quality measurements for 10 clusters harvested from each treatment plot on Sept. 18th, 2008. |
Berries were removed from each of the clusters and the number dehydrated berries and berries damaged by insect feeding were each tallied. Dehydrated, shriveled berries occurred in significantly higher frequency in control plots compared with buckwheat or irrigated plots. The number of berries with broken skin from insect damage was up to 1811% higher in buckwheat plots than in both irrigation plots and controls. This suggests that the nectar provided by the buckwheat plots may have attracted insects, such as bees and yellow jackets, which then fed on ripened berries. The percentage of scarred berries (indicating feeding by thrips adults and larvae) was 38% higher in buckwheat plots compared with controls. Healthy berries were randomly selected from each cluster to be measured using digital calipers (Diagram 6). Berry size was equivalent for all treatments.
Vine Vigor |
The influence of cover crops on vine vigor was investigated in October 2008 by measuring the weight of winter prunings from three randomly selected vines at the center of each treatment plot. The number of canes growing from each arm was recorded for each vine, then all canes were harvested and striped of all leaves and secondary cane growth. The average weight per cane was calculated in the laboratory, and it was determined that canes harvested from buckwheat and irrigation treatment plots weighed 63-69% more than canes from control plots. This difference can be attributed to the extra irrigation the vines received.
Dispersal of Natural Enemies |
In July 2008, a trial to investigate the dispersal of natural enemies from cover crop plots into the vineyard was conducted. Insects in four buckwheat treatment plots were triple-marked with a solution of yellow fluorescent SARDI pigment, milk, and egg whites by spraying the cover crop plants with a backpack sprayer, and captured using additional transparent sticky cards placed at intervals in rows adjacent to the treated plots. These traps were then scanned with a UV light to detect the presence of the fluorescent paint on beneficial insects. All marked beneficial insects were removed, along with a percentage of the unmarked insects, and sent to Dr. James Hagler (USDA-ARS Phoenix, AZ) for ELISA testing to detect milk and egg proteins. Statistical analyses of these results are currently being conducted.
The information gained from this trial will help establish the number of rows of cover crops growers would need to sow to ensure the adequate dispersal of biological control agents from resource-providing plants. Data from this experiment will be used to determine how far marked beneficial insects disperse from cover crop refuges, how many cover crop rows are required to assist natural enemy dispersal in vineyards, and whether control plots contained marked insects from neighboring cover crop treatments.
Grape Pathogens, Pathogen Vectors, and Grape Pests |
Laboratory trials were conducted to determine if buckwheat and vetch plants could act as a host for X. fastidiosa, the causative agent of Pierce’s Disease (PD) in grapes, and if GWSS could transmit X. fastidiosa from these cover crop plants to grapes. If GWSS can transfer the pathogen from the cover crop to the grapes, then these plants may act as a reservoir for disease within the vineyard and be detrimental to growers.
Twenty-five buckwheat and vetch plants were needle-inoculated with X. fastidiosa and tested with ELISA kits after four weeks for the presence of the disease. Results of these kits showed that X. fastidiosa can successfully infect and replicate in both plant species. Further testing showed that GWSS can transmit X. fastidiosa between buckwheat plants, from buckwheat to grapevines, and between grapevines. Greenhouse transmission results for vetch plants were inconclusive, but field trials conducted at UCR AgOps showed positive results for transmission of X. fastidiosa from grapevine to both buckwheat and vetch.
References |
- Altieri, M. A., Schmidt, L. L., 1985. Cover crop manipulation in Northern California orchards and vineyards: effects on arthropod communities. Biological Agriculture and Horticulture 3, 1-24.
- Costello, M. J., Daane, K. M., 1998a. Effects of cover cropping on pest management: Arthropods. In: Cover Cropping in Vineyards: A Grower’s Handbook. Ingels, C. A., Bugg, R. L., McGourty, G. T., Christensen, L. P. (eds.), University of California, Division of Agriculture and Natural Resources. Publication 3338. pp. 93-106. . In 1993-94 Research report for California table grapes. Fresno: California Table Grape Commission.
- Costello, M. J., Daane, K. M., 1998b. Can cover crops reduce leafhopper abundance in vineyards? California Agriculture, 52(5), 27-33.
- Dlott, J., Ohmart, C. P., Garn, J., Birdseye, K., Ross, K., (eds.) 2002. The Code of Sustainable Winegrowing Practices Workbook. Wine Institute and California Association of Winegrape Growers. 477 pp.
- English-Loeb, G., Rhainds, M., Martinson, T., Ugine, T., 2003. Influence of flowering cover crops on Anagrus parasitoids (Hymenoptera: Mymaridae) and Erythoneura leafhoppers (Homoptera: Cicadellidae) in New York vineyards. Agricultural and Forest Entomology 5, 173-181.
- Hanna R., Zalom, F. G., Elmore, C. L., 1996. Integrating cover crops into vineyards. Grape Grower 27(1), 26-43.
- Irvin, N. A., Hoddle, M. S., 2007. Evaluation of floral resources for enhancement of fitness of Gonatocerus ashmeadi, an egg parasitoid of the glassy-winged sharpshooter, Homalodisca vitripennis. 40 (1). 80-88.
- Klonsky, K., Zalom, F., Chandler, M., Ohmart, C., Elmore, C., Tourte, L., 1998. The Lodi-Woodbridge Winegrape Commission: a framework for implementing IPM. Integrated Pest Management Reviews 3, 243-255.
- McKendry, M. V., 1992. Cover crops and nematode species. KAC Plant Protection Quarterly 2(4), 4-8.
- Nagarkatti, S., Tobin, P. C., Saunders, M. C., Muza, A. J., 2003. Release of native Trichogramma minutum to control grape berry moth. Canadian Entomologist 135(4), 589-598.
- Nicholls, C. I., Parrella, M. P., Altieri, M. A., 2002. Reducing the abundance of leafhoppers and thrips in a northern California organic vineyard through maintenance of full season floral diversity with summer crops. Agricultural and Forest Entomology 2, 107-113.
- Settle, W. H., Wilson, L. T., Flaherty, D. L., English-Loeb, G., 1986. The variegated leafhopper, an increasing pest of grapes. California Agriculture 40, 30-32.