Classical Biological Control of Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Liviidae), in California
Table of Contents
1 Introduction
1.1 The Problems Caused by Invasive Pests
1.2 The Asian Citrus Psyllid
1.3 Distribution of ACP and CLas in the USA
2 Economic Impacts
3 The ACP-CLas Situation in California
4 Identification, Biology, and Ecology of ACP
4.1 Eggs
4.2 Nymphs
4.3 Adults
5 CLas Acquisition and Transmission
6 CLas Pathology and Detection
7 Challenges of Controlling ACP-CLas in California
8 The Need for Biological Control of ACP in California
9 Development of a Classical Biological Control Program for ACP in California
9.1 Tamarixia radiata
9.2 Release of Tamarixia radiata in California
9.3 Diaphorencyrtus aligarhensis
9.4 Release of D. aligarhensis in California
9.5 Hyperparasitoids
10 Generalist Natural Enemies, Invasive Ants, and their Impacts on ACP Biological Control in California
11 References
Introduction: The Problems Caused by Invasive Herbivorous Insect Pests
| Figure 1. Adult Asian citrus psyllid. (Photo: Mike Lewis, Center of Invasive Species Research, UC Riverside). |
Compared to their counterparts in the native range, insect herbivores that are considered invasive tend to have populations that are more abundant and damaging in the non-native range in which they successfully establish (Strong & Pemberton 2000; Hoddle 2004; Gandhi & Herms 2010). The “enemy-free” environment of the invaded area, an abundance of host plants, and permissive climate are considered key factors in facilitating the success of exotic phytophagous insects that become pests (Messing & Wright 2006). These factors, along with high phenotypic plasticity and broad ecological tolerances may also facilitate pestiferousness in agricultural and native ecosystems (Lodge et al. 2006; Davidson et al. 2011). In turn, problems associated with the introduction of alien species may be immediate and severe or populations could undergo a prolonged “lag period” before reaching pest status (Crooks et al. 1999; Sakai et al. 2001). Once established, pest herbivores exert strong negative impacts on agroecosystems which often have negative economic outcomes (Hoddle 2004; Goldson et al. 2005).
The Asian Citrus Psyllid – Candidatus Liberibacter asiaticus Pathosystem
One particularly devastating exotic pest of citrus in California is the Asian citrus psyllid (ACP), Diaphorina citri Kuwayama (Hemiptera: Liviidae) (Figs. 1-3) (Hoddle and Pandey 2014; Bistline-East et al. 2015; Bistline-East and Hoddle 2016). D. citri is thought to be native to the Indian subcontinent (Halbert and Manjunath 2004; Beattie et al. 2009) and it has emerged as the most important sap-feeding insect pest of citrus throughout the world (Asia, Middle East, South and Central America, Mexico, United States and the Caribbean) (Bové 2006; Wang et al. 2006; Hall et al. 2013; Grafton-Cardwell et al. 2013). Damage caused by ACP feeding on citrus phloem sap is two-fold (Halbert and Manjunath 2004). First, psyllid feeding can directly damage young citrus foliage (sometimes referred to as flush growth). This occurs because salivary toxins that ACP injects during the process of feeding deforms the tips of young leaves causing them to readily break off (Halbert and Manjunath 2004; Hall et al. 2013). Second, and more importantly, ACP vectors a phloem-limited bacterium, Candidatus Liberibacter asiaticus (CLas), a causative agent of a lethal, incurable, and currently untreatable citrus disease, huanglongbing (HLB),also known as ‘citrus greening disease’ (Graça and Korsten 2004; Bové 2006; Pelz-Stelinski et al. 2010). Characteristic symptoms associated with the HLB disease are shoot and leaf yellowing and mottling, fruit asymmetry, small fruit with bitter juice, premature fruit drop, seed malformation, overall yield reductions, and premature death of citrus trees (Graça and Korsten 2004; Bové 2006). Once infected with the CLas, trees can, however, stay asymptomatic for up to 2 years making CLas extremely difficult to detect in the early stages of disease development (Halbert and Manjunath 2004). The majority of commercially available citrus varieties and closely related rutaceous plants are affected by the ACP-HLB complex. There are almost no exceptions to vulnerability to infection by CLas but disease severity can vary amongst citrus varieties (Yang et al. 2006; Grafton-Cardwell et al. 2013).
Distribution of ACP and CLas in the USA
ACP and CLas (manifested as HLB) were first detected in the United States (US) in Florida (FL) in 1998 and 2005, respectively (Halbert and Manjunath 2004; Hall et al. 2013). Since then, the ACP-CLas pathosystem rapidly spread throughout FL and a major conduit for ACP dispersal was on nursery stock, especially unregulated movement of ornamental sweet orange jasmine, Murraya paniculata (L.). More than 75% of Florida’s citrus trees are infected with CLas and all commercial production areas have trees exhibiting HLB symptoms (Bové 2006; Hodges and Spreen 2012). As of 2016, ACP has been detected in 10 US states (AL, AZ, CA, FL, GA, HI, LA, MS, SC, TX), while CLas is recorded from six states (CA, FL, GA, LA, SC, TX) (Grafton-Cardwell et al. 2013). Thus, HLB is currently considered the most important vector-borne disease threat to citrus industry in the US (Hall et al. 2013; Grafton-Cardwell et al. 2013; Ukuda-Hosokawa et al. 2015).
Economic Impacts
|
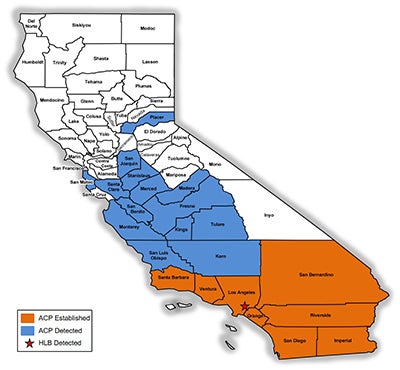
Figure 2. Known distribution of ACP in California as of January 2017. |
The economic impacts of CLas spread by D. citri in Florida have been catastrophic. Losses attributable to ACP-CLas have been estimated at $3.6 billion in net losses with over 8,000 jobs lost, and 33% increase in production costs (e.g., ACP management) (Spreen et al. 2014), and productive acreage has declined by 44% (from a high of 815 100 acres in 1996 to 459 100 acres in 2015) (Hodges and Spreen 2012; Keremane et al. 2015; USDA NASS 2016a).
As of 2017, the HLB situation in California is still developing. ACP is spreading through major citrus growing areas (Civerolo 2015) and CLas is not widely established, being confined to urban areas in Los Angeles County (Hacienda Heights; [detected 2012; Kumagai et al. 2013]) and San Gabriel [detected 2015; Mohan 2015]) (FIg. 2) (CDFA 2016). With recent examples of HLB-mediated citrus decline in Florida and Texas and the billions of dollars attributed to ACP-CLas control and HLB-induced decline of citrus production, it is abundantly apparent that California’s $3 billion-a-year citrus industry is threatened by ACP-CLas.
California is the second largest producer of citrus after FL in the US (i.e., CA was responsible for half of total citrus production in 2014-2015) but first in fresh-market production and export, supplying more than 85% of the country’s whole oranges and tangerines, and over 90% of whole lemons (USDA NASS 2016a,b). Considering the destination of product is critical; unlike Florida’s juice crop which is now chemically altered during processing to mask flavor changes, there is no tolerance for malformed, bitter fruit in California’s predominantly fresh-market crop (Bennet 2016). Declines in California’s citrus production and quality would almost certainly reverberate into the fruit processing industry which employees 26,000 people (Richards et al. 2014). California growers are already bearing the burden of increased ACP-HLB management costs and sponsorship of urgent research needs, and economic forecasts predict that pesticide and nutrition costs may rise to $220 million per year in the next five years (Bennet 2016).
The ACP-CLas Situation in California
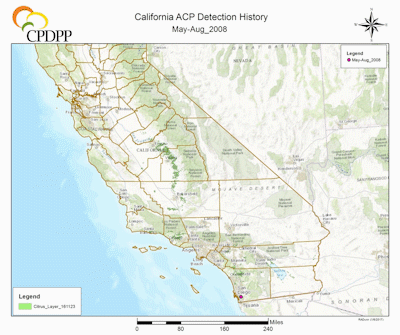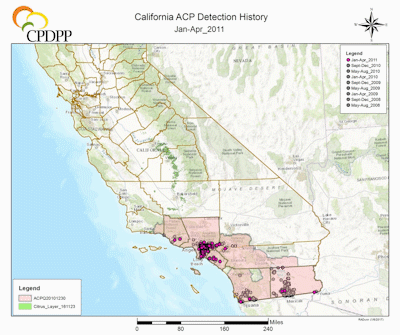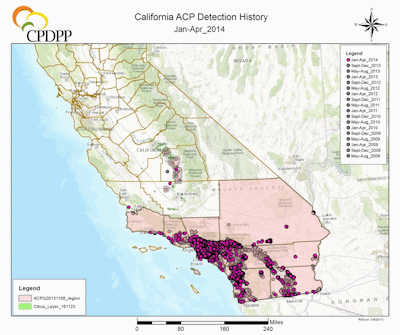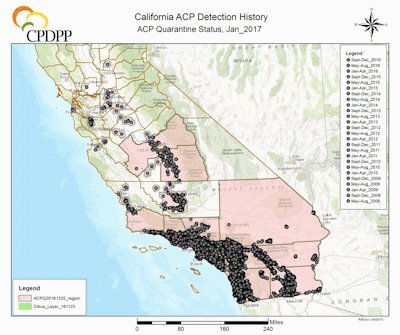
|
ACP Detection History in California. (Maps courtesy of Rick Dunn, Citrus Research Board Director of Data and Information Management, Visalia, CA). |
The first ACP detection in California was recorded in 2008 in San Diego County, in close proximity to the US border with Mexico, a most likely source of psyllid infestation as ACP was widespread in Mexico and probably naturally expanding its range north (Hoddle et al. 2014; Kistner et al. 2016a). Shortly after the detections in San Diego County, large populations were detected 185 km (115 mi) north in Los Angeles (LA) County. The lack of ACP populations on citrus, which was abundant between these two find sites strongly suggests two separate introductions of ACP had occurred in southern California, given the large size of the LA populations in comparison to those in San Diego County. Subsequently, ACP populations have been detected in at least 22 counties in California (CDFA 2016), including the San Joaquin Valley where ~77% of California’s citrus industry is situated (Fig. 2) (USDA NASS 2016b). As of 2017, the official distribution of CLas in California is restricted to a range of 25 km in radius from the original source of detection which includes the first CLas infected tree was discovered in Hacienda Heights, Los Angeles County, in 2012 (Fig. 2) (Leavitt 2012; Hornbaker & Kumagai 2016; Kumagai et al. 2013). Although the current establishment of D. citri is still largely confined to urban-residential areas (Fig. 2) (CDFA 2016), there is a well-recognized risk of psyllid migration into commercial citrus production areas. If the ACP-CLas complex establishes in major citrus growing areas of California, economic impacts could be devastating (see section above for more details on the economic impacts of ACP-CLas on citrus production).
Identification, Biology, and Ecology of ACP
D. citri has a relatively simple ‘hemimetabolous’life cycle. The ACP life cycle consists of an egg, 5 nymphal instars (each time nymphs molt they grow a little larger and their wing pads enlarge and each progressive stage in the molting cycle is referred to as an instar), and a winged adult stage (Grafton-Cardwell et al. 2013) (Fig. 3[I3] ). A typical life cycle (i.e., egg to adult) lasts from 15-47 days (Halbert & Manjunath 2004), but can vary greatly depending upon environmental conditions (temperature: Halbert & Manjunath 2004; relative humidity: McFarland & Hoy 2001), and host plant species (Liu & Tsai 2000).
Eggs. ACP eggs are 0.3 mm long, elongated with a tapered tip, and they are attached to the surface of the host plant with a short stalk or pedicel, which is approximately 0.04 mm long. The long axis of the egg is vertical to plant surface which gives the egg the appearance of standing on its end (Hall et al. 2013) (Fig. 3a,b). Freshly deposited eggs are pale, but gradually turn bright yellow and later orange before hatching. Eggs are laid only on young plant tissue, most abundantly on the unfolding apical ‘feather flush’ leaves (Fig. 4[I4] ) (Halbert & Manjunath 2004). Fertilized eggs typically take from 2-4 days to hatch, and unfertilized eggs do not hatch (Tsai & Liu 2000; Hall et al. 2013).
|
Figure 4. Citrus ‘feather flush’ leaves: orange (a,b,d), lemon (c). (Photos: Mike Lewis, Center of Invasive Species Research, UC Riverside). |
|
Nymphs. The adult stage is preceded by 5 nymphal instars each similar in appearance, but increasing in size after each molt (0.25-1.7 mm long) (Fig. 3b-e) (Grafton-Cardwell et al. 2013). Early instars are pale and as development progresses the color changes to orange-brown (Fig. 3b-e). D. citri nymphs lack fully developed wings, although non-functional wing pads are prominent in late stage instars (Fig. 3c-e) (Hall et al. 2013). Older nymphs (i.e., third through fifth instars) are mobile and walk to new sites to feed and to find protected places to undergo the final molt to the adult stage. In comparison, instars 1-2 tend not to be as motile as the larger nymphs (Tsai & Liu 2000; Grafton-Cardwell 2005). Nymphs feed on phloem sap.
Environmental conditions and host plant can influence nymphal development. Optimal development occurs at 25-28oC and nymphal stages last from 11-15 days (Liu & Tsai 2000). Like eggs, nymphal survival is limited to tender young tissue; thus, the greatest natural mortality occurs at these stages (Hall et al. 2013). Visual inspection of new growth is needed to detect both ACP eggs and nymphal stages. Feeding nymphs excrete honeydew and produce distinguishable curled white waxy tubules, which is a unique visual cue for rapidly identifying colonies of ACP on citrus flush (Fig. 3b-e) (Grafton-Cardwell 2005).
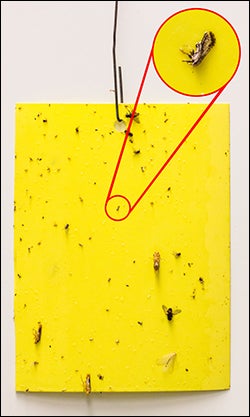
Adults. Adult ACP are small, ~ 4 mm long, with a mottled gray body covered with white waxy secretions and pale brown antennae with black tips (Figs. 1 & 3f) (Hall et al. 2013). After the last nymphal molt, adults are at first pale brown-gray, but soon darken (Grafton-Cardwell et al. 2013). The distinctively patterned forewings of D. citri are broadest at the back (Figs. 1 & 3f). Adult D. citrifeed on phloem plant tissue, primarily on the underside of citrus leaves or new growth. When feeding, adult ACP hold their bodies in a characteristic head down-tail up posture, which is achieved by resting on the “elbows” of bent front legs which creates ~45o angle with the plant (Figs. 1 & 3f) (Hall et al. 2013). In the field, adult ACP can be found on citrus via visual inspection of new flush, tap sampling or by captures on yellow sticky cards (Fig. 5[I5] ).
|
Figure 5. Yellow sticky trap with ACP captured on it. (Photo: Mike Lewis, Center of Invasive Species Research, UC Riverside). |
The abdominal color can vary significantly among individual adults. Three types of adults with different morphology (i.e., morphotypes) are recognized: gray/brown, blue/green, and orange/yellow (Wenninger & Hall 2008; Wenninger et al. 2009). These morphotypes have been associated with different biological and functional traits (Martini et al. 2014). For example, orange-yellow morphs are present only in older males (> 30 d after adult emergence) and gravid females (Wenninger and Hall 2008). Orange-yellow morphs tend to exhibit high susceptibility to insecticides (Tiwari et al. 2013). Blue-green individuals tend to have greater fitness, flight capability, and reproductive potential than other two morphotypes (Wenninger & Hall 2008, Wenninger et al. 2009).
D. citri adults are active, being able to jump and fly. Flight capabilities, are generally weak (Sakamaki 2005; Arakawa & Mivamolo 2007). However, Martini et al. (2014) identified a maximum dispersal distance of 2.4 km (~3h of continuous flight) for a single flight by an individual green-blue morph. Long-distance migration by D. citri most likely requires combination of wind assistance (Martini et al. 2014) and short-distance flights between managed and unmanaged groves (Boina et al. 2009). Unintentional long distance dispersal of adult and immature ACP by humans, most likely through the movement of infested plant material or by hitch-hiking in fruit bins, has probably been the major conduit for movement into new areas (Halbert et al. 2010)
The adult life span can last for several months, however longevity of ACP varies greatly with environmental conditions (temperature: Liu & Tsai 2000; relative humidity: McFarland & Hoy 2001), host plant (Richardson & Hall 2012; Hall et al. 2013), and sex (Nava et al. 2007). Optimal adult longevity occurs at 24-28oC (Liu & Tsai 2000; Fung & Chen 2006). On average, D. citri females live longer (31-32 days at 24oC) than males (21-25 days at 24oC) (Nava et al. 2007).
Adult males and females become reproductively mature within 2-3 days after the last nymphal molt, with a pre-oviposition period (i.e., the time that passes before egg laying can commence) of 1-2 days (Wenninger & Hall 2007). Females copulate with multiple partners throughout and multiple matings with different males promotes enhanced reproductive output (Wenninger & Hal 2008). Adult females can lay on average 500-800 eggs over a lifetime (Tsai & Liu 2000; Nava et al. 2007). Optimal oviposition occurs at 28-30oC (lower temperature threshold: 16oC; upper temperature threshold: 42oC) (Hall et al. 2011), and relative humidity of 45-55% (Skelley and Hoy 2004).The fecundity of D. citri, however, significantly decreases with relative humidity below 40% (Skelley and Hoy 2004).
CLas Acquisition and Transmission
Three closely related species of phloem-limited, alphaproteobacteria, Candidatus Liberibacter ‘asiaticus’(CLas), ‘Ca. L. africanus’, and ‘Ca. L. americanus’, and their insect vectors, ACP and African citrus psyllid, Trioza erytreae (Del Guercio) (Hemiptera: Triozidae) are responsible for the global HLB epidemic affecting more than 40 countries located within Asia, Africa, the Americas (Bové 2006), and parts of the Mediterranean (Pérez-Otero et al. 2015; Monzó et al. 2015). HLB is an incurable, lethal disease of all commercial citrus cultivars and many related species in the family Rutaceae (Halbert & Manjunath 2004; Bové 2006). The majority of these cases, including those found in California, are caused by CLas vectored by ACP (Gottwald 2007, Hall & Gottwald, 2011). Owing to a lack of sufficient vector control, HLB incidence and severity continues to rise in all areas of establishment, threatening the profitability of citrus industries worldwide (Gottwald 2010).
The CLastransmissioncycle is a multi-step process. Acquisition occurs when a psyllid of the adult or nymphal life stage feeds on the phloem of an infected host plant with its piercing-sucking mouthparts. Following feeding, CLas enters the alimentary canal and salivary glands where it replicates (Gottwald 2010), though it is capable of colonizing most psyllid tissues and may be found systemically (Ammar et al. 2011). Following a latency period ranging from 1-20 days, the bacterial titer of CLas in the psyllid rises to a transmittable level (Roistacher 1991; Sakamaki 2005; Pelz-Stelinski et al. 2010). Finally, infected psyllids inoculate plants with CLas during a feeding duration of at least one hour (Xu et al. 1988; Pelz-Stelinski et al. 2010). During this feeding period, bacteria migrate from the salivary glands in saliva and are “injected” into the plant through the insect’s piercing-sucking mouthparts (Ammar et al. 2011). After bacterial titers rise to infectious levels in plant parts that psyllids feed on, infected and uninfected psyllids may pick up CLas through feeding and repeat the inoculation cycle again.
Acquisition of CLas from infected plants by nymph and adultstage ACP can occur in 24 hours (Roistacher 1991; Inoue et al. 2009; Pelz-Stelinski et al. 2010), though the rate increases with longer feeding duration and increasing plant bacterial titer (Pelz-Stelinski et al. 2010). Plant-bacterial titer generally rises with increasing time since initial plant inoculation with CLas, proximity to infection location(s), and an increasing number of points of infection (due to multiple feeding psyllids) (Pelz-Stelinski et al. 2010). Under facilitative circumstances, plant CLas titer can reach a level transmissible to ACP just 10 to 15 days after initial inoculation by an infected adult ACP (Lee et al. 2015).
Though CLas acquisition is possible in both the nymphal and adult stage, the rate is higher fornymphs (Pelz-Stelinski et al. 2010). Infections acquired as nymphs are retained as adults (Pelz-Stelinski et al. 2010). CLas-infected nymphs have a shorter lifespan as adults (Pelz-Stelinski & Killiny 2016) and are more susceptible to major classes of insecticides than uninfected nymphs (Tiwari et al. 2011). However, CLas-infected nymphs develop more quickly, and as adults, have a higher reproductive rate, translating into greater vector population growth in infected areas and concomitant CLas spread (Pelz-Stelinski & Killiny 2016). The overall beneficial effects of CLas on their ACP vectors means they likely have had a long, stable co-evolutionary history that developed prior to development of CLas as a plant pathogen (Purcell 1982).
In addition to acquisition from feeding on infected plant material, ACP may acquire CLas from other ACP. Both transovarial (female to offspring) transmission (Pelz-Stelinski et al. 2010) and male to female sexual transmission of CLas occur, albeit at low rates (~ 3%) (Mann et al. 2012). Because of the high fecundity of ACP and the potential for this pest to develop high density populations (Fung & Chen 2006; Hall et al. 2008), these secondary transmission routes may allow CLas to persist in ACP populations in the absence of plant inoculum sources (Grafton-Cardwell et al. 2013).
The results of studies which examine whether or not continual feeding is circulative (i.e., infected plant material is not required for ACP to remain infectious throughout their lives) are conflicting. Though the bacterial titer of infections derived from both nymph and adult ACP stages decreases throughout the insect’s lifetime in the absence of an inoculum source (Pelz-Stelinski et al. 2010), they may still remain infectious throughout their life (Xu et al. 1988; Hung et al. 2004). A threshold for the necessary bacterial titer in ACP required for transmission to an uninfected plant has not been firmly established because there are so many contributing factors.
Studies have reported highly variable transmission rates of CLas by ACP with values ranging from 1.3-80% (Huang et al. 1984; Xu et al. 1988; Inoue et al. 2009; Pelz-Stelinski et al. 2010). Several factors contribute to this inconsistency, including host plant variety and age (younger hosts are more susceptible) (Xu et al. 1988; Gottwald 2010), patchy CLas distribution and titer in the plant (which may confound detection) (Teixeira et al. 2008; Gottwald 2010), CLas detection techniques employed (i.e., PCR or visible symptoms) (Hall et al. 2012), ACP life stage in which infection was acquired (i.e., transmission rate is higher for adult ACP which acquired CLas as nymphs vs. adult-only acquisition) (Inoue et al. 2009; Pelz-Stelinski et al. 2010; Ammar et al. 2011), number and duration of infected ACP feeding (Xu et al. 1988; Pelz-Stelinski et al. 2010; Coletta-Filho et al. 2014), and differences in experimental design. However, both model simulations and field observations suggest that CLas can spread rapidly through ACP populations and citrus groves (Xu et al. 1988; Hall et al. 2013; Lee et al. 2015).
One important contributing factor to rapid spread of CLas is the high dispersal potential of ACP. Dispersal is facilitated by their small size (they can be blown by wind), high reproductive output, large population densities, substantial flight capabilities (2 km in 12 days and across geographical barriers such as roads and fallow fields), and a propensity to spread through human assisted movement of infested plants (Fung & Chen 2006; Hall et al. 2008; Khan et al. 2014; Lee et al. 2015; Lewis-Rosenblum et al. 2015). Additionally, uninfected adult ACP are more attracted to CLas infected trees, and following CLas acquisition, to uninfected trees, a pattern which may further promote pathogen acquisition and spread (Mann et al. 2012). In model simulations, just 600 CLas infected adult ACP placed on trees in the center of a citrusgrove were sufficient to infect the entire citrus grove in less than a year (Lee et al. 2015). Complete infection from grove edges was possible with ~1,000 infected ACP. However, with 75% or 90% ACP population reduction, CLas spread and healthy fruit production can be extended by a year or several, respectively (Lee et al. 2015). Thus, vector control is critical to reduce CLas movement into uninfected groves and improve longevity and productivity of trees within already infected groves.
CLas Pathology and Detection
|
Figure 6. Citrus greening disease: mottled leaves (a), soon-to-be dead tree (b). (Photos: Dr. Elizabeth Grafton-Cardwell, UC Riverside). |
Following plant acquisition of CLas, tissues undergo a series of physiological and metabolic changes which manifest as reduced vigor, foliar discoloration and dieback, stunted growth, premature fruit drop and diminished or unmarketable production (i.e., fruit are green, small, and misshapen, with aborted seeds and a bitter flavor), and eventual tree death (Fig. 6[I6] ) (Etxeberria et al. 2009; Brodersen et al. 2014). Infection is initially localized, and within affected leaves and limbs the phloem tissue degenerates, leading to blockages and collapse (Brodersen et al. 2014). As the infection spreads systemically (first downward with phloem flow to the roots and later to other canopy regions [Graham et al. 2013]), nutrient transfer is compromised, resulting in deficiencies in nitrogen, phosphorus, iron, zinc, magnesium (Mann et al. 2012), amino acids (da Graça 1991), and, as a result of root decline, water and carbohydrates (Johnson et al. 2014). The necrosis of phloem tissue also increases susceptibility to pests and secondary infections such as root and fruit rots caused by Phytophthora species (Graham et al. 2013; Graham 2016). Despite infection, trees may remain productive for years due to root growth stimulated by root decline (Graham 2016) and cycles of flushing during which new vegetative and reproductive growth with healthy phloem tissue is produced (Ikpechukwu et al. 2011; Brodersen et al. 2014). Ultimately, however, fruit quality and quantity is diminished and trees becomeunproductive. Tree death typically occurs within ten years post-infection with CLas (Bové 2006; Gottwald 2010). Field observations indicate orchards may become economically unfeasible five to ten years after infection of the first tree or two to five years if the orchard was infected when trees were under five years of age (Bassanezi & Bassanezi 2008), though this timeline may be impacted by several parameters such as infected psyllid density and spatial distribution of ACP and CLas in groves (reviewed in Lee et al. 2015).
Confirmed HLB Finds
|
||
| Year | County | # of Sites |
| 2012 | LA | 1 |
| 2013 | N/A | 0 |
| 2014 | N/A | 0 |
| 2015 | LA, Orange | 12 |
| 2016 | LA | 22 |
| 2017 | LA, Orange | 26 (as of 6/2017) |
|
HLB was found on the following citrus varieties: Lemon, Orange, Kumquat, Lime, Calamondin, Grapefruit, Mandarin, and Pummelo. |
||
The pathology of CLas has critical implications for disease control. Though symptoms may not manifest visibly for several years (Gottwald 2010), plants can be active sources of inoculum for disease spread throughout this time (Coletta-Filho et al. 2014; Lee et al. 2015). Because of this, symptom onset is a poor indicator of CLas infection in a grove. Unfortunately, early and accurate molecular field detection techniques for CLas are lacking. PCR is cost and time prohibitive for monitoring and may yield false negatives (i.e., results that indicate a tree is not infected with CLas when it is infected) due to multiple infection foci and discontinuous bacterial distribution and titers (Gottwald 2010). Though CLas detection is more consistent in roots than leaves, root sampling is very labor-intensive and unfeasible at a large scale (Johnson et al. 2014). User-friendly detection kits and new detection methodologies currently under development are promising (Keremane et al. 2015; Pourreza et al. 2015; Wetterich et al. 2016) but not yet commercially available. Even following positive CLas detections, many growers are hesitant to remove infected but still productive trees due to economic loss and an unwillingness to acknowledge the significant risks associated with maintaining diseased trees in orchards (Spann et al. 2011; Hall et al. 2013).
Challenges of Controlling ACP-CLas in California
Management of invasive pests aims to prevent their establishment and should establishment occur, minimizing rate of spread and population growth is important (Messing & Wright 2006). Because ACP populations in CA are widespread in both urban and agricultural areas, a multifaceted approach that incorporates chemical, cultural, and biological control is necessary for successful and sustainable vector management (Qureshi & Stansly 2007). Following early detections of ACP, the California Department of Food and Agriculture (CDFA) initiated urban chemical (i.e., imidacloprid soil drenches and cyfluthrin foliar sprays) and monitoring programs in attempt to eradicate ACP (Hoddle 2012). The CDFA spray program was expensive, at ~$200 million (treatments cost more than $100 per residence), <10% of urban properties with citrus at risk of infestation by ACP were treated with pesticides, and the program was terminated in 2012 after 3 years, in part, because of cost and increasing detections of ACP outside of treatment areas (Hoddle and Pandey 2014). Despite these extensive efforts to suppress ACP in urban areas, this pest rapidly spread throughout southern California, subsequently expanding into the Central Coast (2009), Central Valley (2012), and the greater San Francisco Bay Area (2014) (Fig. 2) (CDFA 2016).
As of January 2017, the CDFA-appointed ACP quarantine spanned 22 counties, encompassing over a third of California (i.e., 160,282 square kilometers [= 61,900 square miles]). Quarantines restrict movement of citrus and curry plant nursery stock outside designated areas, unless grown within a screened USDA-approved facility (CDFA 2016).The Citrus Health Response Program (CHRP) pertaining to fruit harvesting, packing, and processing has been developed by the USDA to reduce the likelihood of pest-disease spread in affected areas, including California (USDA CHRP 2016). Practices such as pre-harvest grove surveys, worker education, inspection of fruit in field bins, bin hygiene and debris removal, washing and waxing of fruit, equipment sanitation and proper storage and shipping procedures may reduce the likelihood of fruit processing practices accidentally moving ACP into new areas.
One likely reason for the slower expansion of ACP through California in comparison to Florida may be due, in part, to these recommended quarantine practices. As of January 2017, California citrus (both backyard and commercial) has not yet suffered from the widespread establishment of CLas. Minimizing the rate of spread of CLas through vector (i.e., ACP) reduction is a very important citrus industry priority.
The Need for Biological Control of ACP in California
Reduction of ACP populations by biological control agents can increase the efficacy and sustainability of other control strategies, including pesticide use, because fewer ACP need to be managed less frequently. In California, biological control of ACP is the primary control option in areas where pesticide use is limited or not feasible, such as urban environments, organic, or abandoned citrus orchards. Urban citrus represents a large proportion of total citrus in California. In Los Angeles County alone, there are an estimated 1.23 million residences with at least one citrus tree (Hoddle 2012). Though large scale urban chemical control programs are prohibitively expensive (see above), ACP suppression is urgently needed because garden citrus harbor the largest populations of ACP and backyard citrus is the only known reservoir for CLas in CA.
Infested residential areas are the most important sources of ACP and CLas from which dispersal to commercial groves can occur. This ongoing pressure, despite best-efforts of growers maintaining rigorous ACP-CLas control programs, increases the likelihood that ACP and CLas will eventually establish populations in commercial organic and conventional citrus orchards (Gottwald 2010).
A similar threat is posed to commercial citrus production from other types of unmanaged citrus such as abandoned citrus orchards (Lewis-Rosenblum et al. 2015).While abandoned citrus is not yet common in California, it may become increasingly so if CLas establishes and associated increases in production costs and decreased profitability result in grower abandonment of citrus groves. Lewis-Rosenblum et al. (2015) demonstrated that abandoned citrus groves in Florida are important disease reservoirs, resulting in movement of infected ACP into managed groves in spring and summer.
Furthermore, control of ACP in unmanaged wilderness areas in California may also be necessary and biological control will be the only feasible option in this instance. There are several species of wild Rutaceae, both Old and New World, that could act as potential hosts for ACP-CLas (Grafton-Cardwell et al. 2013). Native rutaceous plants are widely distributed in California, especially in locations near commercial production areas (Calflora 2016). However, no investigations have been conducted to determine their suitability as reservoirs for ACP-CLas and surveys addressing this possibility should be conducted.
Development of a Classical Biological Control Program for ACP in California
Initial surveys of ACP populations following the discovery of this pest in California revealed that pest densities in urban citrus were high, and the subsequent rearing and dissection of hundreds of field collected nymphs failed to recover parasitoids, a group of natural enemies thought to be important for regulating ACP populations in the native range. This apparent lack of top down control, may have been responsible, in part, for the high numbers of ACP observed in urban citrus, and these large populations, may have been contributing to the rapid spread of ACP in southern California. With the abandonment by CDFA of urban spray programs for ACP control (see above for details on this spray program), the opportunity to develop a biological control program targeting ACP presented itself.
A review of Husain and Nath’s (1927) paper on ACP infesting citrus in Punjab (at this time Punjab Province spanned both sides of today’s Pakistan and India border) indicated that there was potentially a rich parasitoid fauna associated with the pest, with perhaps up to nine species of parasitoid attacking this pest. However, just one parasitoid was identified from this study, Tamarixia radiata(see below for more details), and the identities of the remaining eight parasitoid species, if they existed, were not reported. Additionally, Husain and Nath (1927) indicated that there could be hyperparasitoids attacking ACP parasitoids but the identities of these hyperparasitoids, if they did exist, were not provided. An important question that needed answering was this: “what are the identities of the ACP parasitoids referred to by Husain and Nath (1927)?” The classical biological control program targeting ACP in California aimed to answer this question, amongst others (e.g., are there hyperparasitoids attacking ACP parasitoids in the native range?), by studying ACP infesting citrus in Punjab Pakistan. Pakistan was selected for foreign exploration because this area has a good climate match with the Central Valley, a major citrus production area in California (see below formore details). The foreign exploration program for ACP biological control in California was based out of the University of Agriculture, Faisalabad (UAF) Punjab Pakistan.
Beginning in 2010, researchers at the University of California at Riverside (UCR) in collaboration with professors and students in the Department of Entomology at UAF, scientists from the California Department of Food and Agriculture (CDFA) and the United States Department of Agriculture (USDA) collaboratively developed a classical biological control program to suppress ACP populations in California. Classical biological control refers to the practice of returning to the pest’s home range and looking for host specific co-evolved natural enemies that may contribute to population suppression. The enemy release hypothesis (Keane & Crawley 2002) suggests that one important reason non-native insects become pests in a new area is because of escape from natural enemies which regulate populations in the native range where the pest and its enemies evolved together.
The development of a classical biological control program in California required extensive foreign exploration in Punjab Pakistan, rigorous host range and host specificity testing (i.e., safety testing) to ascertain the risk natural enemies posed to non-target species (e.g., native California psyllids), mass production of natural enemies approved for release, and the subsequent widespread release of the ACP’s two primary parasitoids, Tamarixia radiata and Diaphorencyrtus aligarhensis in California (Hoddle 2012). It is important to note here that parasitoids differ from parasites in that a parasitoid kills its host by feeding on it whereas a parasite extracts nutrients from its host without killing it.
|
Figure 7. Tamarixia radiata: male (left), female (right). (Photos: Mike Lewis, Center of Invasive Species Research, UC Riverside). |
|
|
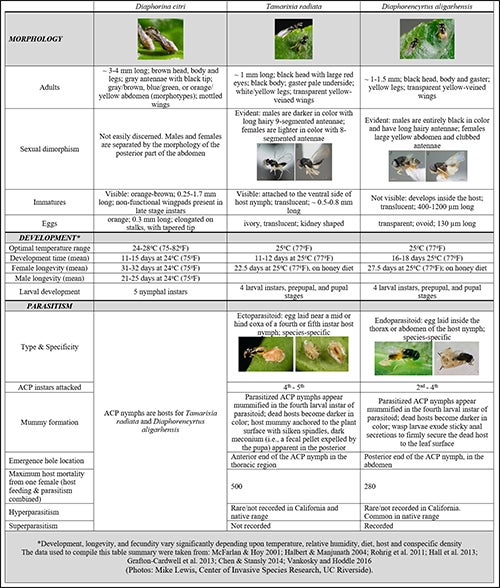
Table 1. Click to view |
|
Tamarixia radiata.(Waterston) (Hymenoptera: Eulophidae) (Fig. 7[I7] &8). Tamarixia radiata is a host-specific, idiobiont ectoparasitoid of ACP nymphs, originally described from specimens that emerged from parasitized ACP nymphs on lemon leaves collected from Lyallpur Pakistan (today this city is known as Faisalabad) (Waterston 1922). T. radiata are small (~1mm long), with a black and yellow body, yellow legs, transparent yellow-veined wings, and two red eyes (Table 1; Fig. 7) (Chen & Stansly 2014). Sexual dimorphism is evident between adult males and females. Males are typically darker and smaller than females, and have long hairy antennae comprised of 9 segments; female antennae are eight-segmented and lack hairs (Fig. 7).
T. radiata females typically lay a single egg near the mid or hind coxa (the leg segment that attaches to the body of the nymph) of a fourth or fifth instar ACP nymph. The egg is attached to the ventral or bottom surface of the ACP nymph (Fig. 8a[I8] ,b) (Vankosky & Hoddle 2016). Here, the parasitoid larva hatches, feeds on the ACP nymph, and develops through four larval instars (Table 1; Fig. 8c,d) over ~11 days at 25oC, and 100% RH (Husain & Nath 1927; Chien et al. 1991; Chen & Stansly 2014). As T. radiata pupate, the appearance of their hollowed out dead hosts ‘mummify’. ACP mummies develop a brown, crusty exterior, beige silken threads that radiate from the margins of the host serve to anchor the ACP shell to the plant, and a dark meconium appears in the posterior end of the host (Fig. 8c-e). The hollowed out host protects the parasitoid pupa. Adult T. radiata upon emerging from the pupa chew a 0.5 mm diameter hole in the anterior thoracic region of the ACP mummy before crawling out (Table 1; Fig. 8e) (Aubert 1987; Chien et al. 1991; Chen & Stansly 2014). In laboratory trials, T. radiata is an efficient parasitoid, inflicting mortality of >90% of presented nymphs (Skelley & Hoy 2004). Chien et al. (1995) estimated that a single female can kill up to 500 nymphs in her lifetime. This is achieved through a combination of parasitism (which accounts for ~80% of ACP mortality), and host feeding (which accounts for about 20% of mortality). Host feeding is the process through which the female uses her ovipositor (an egg laying tube) to mutilate an ACP nymph. Hemolymph leaks from these wounds and female parasitoids consume this fluid which is an important protein source for developing and maturing eggs. The trauma of being “stabbed” and then feed upon is sufficient to kill ACP nymphs.
Both fertilized (result in female offspring) and unfertilized (result male offspring) eggs may be laid immediately following emergence. However, host feeding enables females to produce more eggs over the course of a lifetime, a phenomenon known as synovigeny(Chien et al. 1994; Jervis et al. 2001; Chen & Stansly 2014). Laboratory studies indicate that female T. radiata do not oviposit on previously host-fed nymphs and can discriminate between parasitized and unparasitized hosts thereby avoiding superparasitism(Chen et al. 2016). Overall host-killing capacity (i.e., a function of longevity and fecundity) depends on a variety of factors (see Vankosky & Hoddle 2016) especially temperature, which is optimal at a constant 25oC (77oF) in the laboratory (Chu & Chien 1991; Chien et al. 1995; McFarland & Hoy 2001).
Release of Tamarixia radiata in California. California’s classical biological control program for ACP was initiated in 2010 (Hoddle 2012). T. radiata populations were sourced from Punjab, Pakistan, part of the native range of ACP and an area that was selected, in part, for foreign exploration, because of a 70% CLIMEX match (CLIMEX is a climate modeling program) with major citrus growing regions of California (Hoddle 2012). Biological control theory suggests that climate matching is important in classical biological control because natural enemies should be pre-adapted to the climate in the new area in which release may occur. Six foreign exploration trips to Punjab Pakistan were made over a 2.5 year period. Parasitized ACP nymphs collected from different areas and at different times were used to establish17 female isoline breeding cages in UC Riverside’s quarantine facility (Fig. 9) (Hoddle & Hoddle 2013). Isocage lines were initiated to preserve genetic diversity of collected material based on advice from Dr. Richard Stouthamer (UCR Entomology Professor) and were based on theoretical studies by Hopper et al. 1993 and Roush and Hopper (1995). The basic premise underlying this approach is that parasitoid populations that are allowed to interbreed quickly become adapted to quarantine conditions which is maladaptive for filed establishment.
Although isocage lines become inbred and lose some genetic variability, traits become fixed because of homozygosity. Genetic variation is assumed to be reconstituted to some unknown degree when inbred males and females from each isocage line are introduced into a panmictic mating cage and random male-female pairings result in heterozygote offspring which are released into the field (Fig. 9[I9] ). Natural selection acts on these hybrids selecting for genetic make ups that are best adapted to prevailing conditions in release areas. There is no experimental evidence from laboratory or field studies to suggest that the isocage approach to maintaining genetic diversity of ACP natural enemies imported from Pakistan has benefited the ACP biocontrol program in California. However, the idea theoretical support and is intellectually appealing.
Following the completion of host range and host specificity tests in quarantine and the submission of a report summarizing the results of these studies to USDA-APHIS, permits were issued by USDA-APHIS to release T. radiata from quarantine. Mass production and distribution efforts were taken over from UCR by the CDFA in 2012. As of January 2017, more than 3.5 million T. radiata had been released in southern California by the CDFA (Dr. David Morgan, CDFA 2016 pers. comm.). Releases have resulted in widespread establishment of T. radiata. Evidence of T. radiata activity has been found at approximately 91 percent of surveyed sites (n=100 which included both release and non-release sites), including 86 percent of the non-releases sites that were surveyed (n=28) (Kistner et al. 2016b).
Genetic testing of field recovered T. radiata by Dr. Paul Rugman-Jones in the Stouthamer Lab at UCR indicated that captured parasitoids were of Pakistani origin and sampled populations had almost certainly resulted from releases associated with the classical biological control program targeting ACP in California. Genetic variation of mass produced T. radiata is maintained, to some degree, by introducing parasitoids from the original 17 homozygous isofemale lines from Pakistan into CDFA mass production colonies. In 2017, this approach will change, and T. radiata collected from different geographic areas in California representing different climates (e.g., cool coastal areas [Santa Barbara], moderate intermediate zones [Riverside], and hot interior regions [Coachella Valley]) will be used to initiate new mass production colonies. The rationale underling this change is that natural selection having acted upon the original genetic variation introduced from Pakistan, will have resulted in parasitoid strains best adapted to the prevailing climatic conditions in regions where these natural enemy populations persist.
Post-release monitoring in California indicates ACP parasitism by T. radiata is low to moderate (up to 18.6%), varying greatly across sites, seasons, and years (Kistner et al. 2016a). However, this level of control is significantly higher than what is typically observed in Florida (i.e., 1-3% parasitism) where T. radiata, sourced from Taiwan and south Vietnam (these areas, part of the native range of ACP, have an appropriate climate match for Florida and natural enemies were subsequently sourced from these countries), has also been used for classical biological control of ACP (Qureshi & Stansly 2009).
Several reasons have been proposed for low attack rates by T. radiata on ACP in Florida. First, intensive broad-spectrum insecticide use in citrus production areas (Hall & Nguyen 2010), low genetic variability of released parasitoids (Barr et al. 2009; Chen & Stansly 2014), and interactions with other generalist predators (especially lady bugs), and ant mutualists which protect ACP nymphs from parasitoids (Michaud 2004; Qureshi & Stansly 2009; Navarette et al. 2013).
However, in California (and possibly Florida) the impact of T. radiata on ACP in the field is likely underestimated. Host-feeding, unsuccessful oviposition, and incomplete parasitoid development by T. radiata have been shown to be significant mortality factors for ACP in the laboratory (Chien et al. 1991, 1994) but are almost impossible to quantify in the field while likely contributing significantly to measures of “undetermined” or “unknown” mortality in life table studies (Kistner & Hoddle 2016a). However, use of T. radiata on tropical islands like Reunion and Guadeloupe for biocontrol of ACP has apparently been quite successful ( EPPO 2009 ).
Though commonly used pesticide sprays and residues are known to cause 80-100% mortality of T. radiata within 24-72 h of exposure (Hall & Nguyen 2010), widespread mortality from pesticide use is unlikely in California where the vast majority of T. radiatareleases have been made in urban areas where regular pesticide applications to backyard citrus are assumed to be very low.
|
Figure 10. Diaphorencyrtus aligarhensis: male (left), female (right). (Photos: Mike Lewis, Center of Invasive Species Research, UC Riverside). |
|
Diaphorencyrtus aligarhensis. D. aligarhensis (Shafee, Alam and Argarwal) (Hymenoptera: Encyrtidae) (Figs. 10 [I11] & 11) is a solitary endoparasitoid, highly specific to ACP nymphs, and native to the Indian subcontinent (Yang et al. 2006; Rohrig et al. 2011). D. aligarhensis adults are small insects (~1 to 1.5 mm long), with a black and yellow body and yellow legs, transparent yellow-veined wings, and large gray eyes (Table 1; Fig. 10) (Vankosky & Hoddle 2016). Sexual dimorphism is marked between adult D. aligarhensis males and females. Males have black bodies and long hairy antennae, whereas females have a distinctively yellow abdomen and smooth clubbed antennae (Fig. 10).
Like T. radiata, D. aligarhesis kills its hosts through a combination of parasitism and host feeding (Rohrig et al. 2011). D. aligarhensis attacks second through fourth ACP instars (Skelley & Hoy 2004; Rohrig et al. 2011) and a single female can kill up to 280 nymphs in her lifetime (Chien et al. 1995). Host feeding provides the necessary protein for egg production over the life time of the female (i.e., synovigenic reproduction) (Rohrig et al. 2011). An ovipositing D. aligarhesis female lays a single egg inside the thorax or abdomen of the host nymph (Fig. 11a,b[I12] ) (Vankosky & Hoddle 2016) and superparasitism may occur in some instances (Tang & Wu 1991). Inside the ACP nymph, the parasitoid larva hatches, feeds, and develops through four larval instars killing the host before entering the non-feeding prepupal and pupal stages (Table 1; Fig. 11). Optimal development occurs at 25oC (77oF) and lasts from 16-18 days (Skelley & Hoy 2004; Rohrig et al. 2011). As D. aligarhensis pupate inside the shell of their dead host (referred to as mummies) they become darker in color and parasitoid larvae exude sticky anal secretions to firmly secure the hollowed out cadaver of the dead host to leaf surface (Grafton-Cardwell et al. 2013).
Adult D. aligarhensis chew a 0.5 mm diameter hole in the abdominal region of the mummified ACP through which they emerge from the host cadaver (Table 1; Fig. 11d) (Rohrig et al. 2011). Depending on the source population, D. aligarhensis can be either bi-sexual (e.g., populations from Pakistan, China, Vietnam) or consist only of females (e.g., populations from Taiwan are infected with the intracellular endosymbiont Wolbachia which promotes production of female only offspring) (Skelley & Hoy 2004; Hoddle & Hoddle 2013).The longevity and fecundity of D. aligarhesis are influenced by a variety of abiotic factors (see Vankosky & Hoddle 2016), especially temperature.
Release of D. aligarhensis in California. D. aligarhensis populations for release in California were sourced from Punjab, Pakistan (Hoddle 2012). D. aligarhensis underwent host specificity and host range testing in quarantine at UC Riverside (Bistline-East et al. 2015). Results of these tests demonstrated that D. aligarhensis likely posed little threat to non-target species in California and USDA-APHIS issued a permit authorizing release of the ACP natural enemy from quarantine (Vankosky & Hoddle 2016). A total of five isocage breeding lines (originally nine were established with material collected from Pakistan but four died out because, for unknown reasons, the bi-parental populations turned 100% male) have been maintained to preserve as much genetic diversity as possible (Hoddle & Hoddle 2013). As of January 2017, more than 200,000 D. aligarhensis had been released in southern California by UCR & CDFA (Dr. David Morgan, CDFA 2016 pers. comm.).
The rationale for attempting to establish D. aligarhensis in California was to complement parasitism by T. radiata, something assumed to be possible because laboratory studies by other researchers indicated that these two parasitoids have different host stage preferences, both parasitoid species co-exist in citrus orchards in the native range (i.e., Pakistan), and the possibility exists that differing preferences (e.g., climate, host plants) between D. aligarhensis and T. radiata could result in these natural enemies occupying different citrus production areas in California. Range partitioning by different natural enemy species have been observed previously for other biological control projects targeting citrus pests in California. An example of this is the extremely successful classical biological control of cottony cushion scale, Icerya purchasi Maksell (Hemiptera: Monophlebidae) by the coccinellid Rodolia cardinalis Mulsant and the parasitic fly, Cryptochaetum iceryae (Williston) (Diptera: Cryptochaetidae) (Quezada and DeBach 1973)
D. aligarhensishas failed to establish permanent populations in Florida and this maybe attributable to several factors: negative influence of Wolbachia endosymbionts (causes all female populations thereby eliminating males and the possibility of sexual reproduction which promotes genetic variation) on survivorship, fecundity, offspring sex ratio, and genetic variability of D. aligarhensis (Rohrig et al. 2011), intensive broad-spectrum insecticide use in citrus production areas (Rohrig et al. 2012), lower competitive performance of D. aligarhensis compared to the widely established T. radiata (Skelley and Hoy 2004; Rohrig et al. 2012), and relatively low release numbers which reduces the likelihood of establishment. In comparison to Florida, it is possible that California could provide a better environment for D. aligarhensis establishment and proliferation. First, D. aligarhensis releases in California involve bi-parental populations sourced from Punjab, Pakistan (an area with a 70% climate match with California) which increases genetic variation and the possibility for adaptation (Hoddle & Hoddle 2013), whereas Florida’s biocontrol program for ACP utilizes Wolbachia-infected, thelytokous ecotypes from Taiwan, low in genetic variability because populations are all female (Skelley and Hoy 2004, Rohrig et al. 2012). Second, the majority of California’s releases of D. aligarhensis occur primarily in urban-residential areas where pesticide use is limited, compared to Florida’s releases which are exclusively focused on commercial citrus groves, subjected to intensive pesticide treatments (Vankosky & Hoddle 2016).
There is an increasing experimental evidence from researchers in Florida and California that T. radiata is a superior competitor compared to D. aligarhensis. T. radiata has a significantly shorter generation time, higher reproductive rate, can kill nearly twice as many hosts through a combination of parasitism and host feeding (Skelley and Hoy 2004), and is capable of successfully parasitizing hosts within five days following initial oviposition by D. aligarhensis as T. radiata larvae outcompete D. aligarhensislarvae (Rohrig et al. 2012). In an attempt to mitigate interspecific competition between T. radiata and D. aligarhensis in California, an attempt has been made to release D. aligarhensis in ACP-infested areas that lack T. radiata (e.g., unsprayed urban areas in parts of Santa Barbara County). However, this is proving difficult as T. radiata has spread quickly into non-release areas. One other factor affecting interspecific competition between T. radiata and D. aligarhensis could be heterogeneous climates across major citrus production areas may favor establishment of D. aligarhensis in parts of southern California that could be unfavorable for T. radiata.
Hyperparasitoids. The existence of hyperparasitoids in California may exert strong negative impacts on ACP parasitoids potentially decreasing the efficacy of biological control program (Hoddle et al. 2014). Currently, there is no evidence indicating that T. radiata and D. aligarhensis are subject to high levels of hyperparasitism in California. In contrast to California, hyperparasitoid species are commonly associated with parasitized ACP nymphs, especially those parasitized by D. aligarhensis, in Punjab, Pakistan part of the native range of ACP (Hussain & Nath 1927).
California’s classical biological control program for ACP aimed to reveal the identity of the nine species of parasitoids associated with ACP nymphs as alluded to by Hussain & Nath (1927). To address this issue, a total of 3,675 parasitoids were reared out in quarantine at UCR from material imported under USDA-APHIS permits from Pakistan, and 13 parasitoid species associated with collected ACP were identified (Hoddle et al. 2014). The majority of collected material was represented by T. radiata (55% of collected specimens) and D. aligarhensis (28% of collected specimens.
However, there was a community of 11 other species associated with parasitized ACP nymphs and the small pieces of plant material to which ACP mummies were attached. This community included one lace bug egg parasitoid: Erythmelus panis (Enock) (Hymenoptera: Mymaridae); one leafhopper egg parasitoid: Gonatocerus sp. (Hymenoptera: Mymaridae); three eulophid leafminer parasitoids: Cirrospilus sp., Citrostichus psyllocnistoides, and Sympiesis sp. (all three Hymenoptera: Eulophidae); and five obligate hyperparasitoids of T. radiata and D. aligarhensis: Aprostocetus (Aprostocetus) sp. (Hymenoptera: Eulophidae), Marietta leopardinaMotschulsky (Hymenoptera: Aphelinidae), Chartocerus sp. (Hymenoptera: Signiphoridae), Pachyneuron crassiculme Waterston (Hymenoptera: Pteromalidae), and Psyllaphycus diaphorinae Hayat (Hymenoptera: Encyrtidae) (Hoddle et al. 2013, 2014; Bistline-East & Hoddle 2016). P. diaphorinae was originally described as a primary parasitoid of ACP but subsequent work in quarantine at UCR disproved this (Bistline-East & Hoddle 2016). These rearing records strongly suggest that the guild of nine ACP parasitoid species mentioned by Hussain and Nath (1927) most likely does not exist. It is probable that Husain and Nath (1927) did rear out the two ACP primary parasitoids, T. radiata and D. aligarhensis, mistook hyperparasitoid species as primary parasitoids of ACP, and assumed that other parasitoid species that emerged from unobserved hosts (e.g., insect eggs embedded in plant material) on citrus material were also primary parasitoids of ACP nymphs.
Rigorous quarantine studies at UCR assessing the developmental and reproductive biology of Aprostocetus (Aprostocetus) sp., M. leopardina, Chartocerus sp., P. crassiculme, and P. diaphorinae definitively proved that these species were hyperparasitoids of D. aligarhensis and T. radiata larvae or pupae, and were not ACP parasitoids even though they emerged from ACP mummies. These rearing and biology prevented the unintentional introduction of hyperparasitoids into California (Hoddle et al. 2014; Bistline-East & Hoddle 2016).
Generalist Natural Enemies, Invasive Ants, and their Impacts on ACP Biological Control in California
In addition to parasitism and host feeding by ACP parasitoids, the primary sources of ACP mortality in the field are naturally occurring generalist predators such as lady beetles (Coleoptera: Coccinellidae), syrphid flies (Diptera: Syrphidae), lacewings (Neuroptera: Chrysopidae), and predatory mites (Acari) (Michaud & Olsen 2004; Michaud 2004; Qureshi & Stansly 2007; Juan-Blasco et al. 2012; Kistner et al. 2016b). The extent of control provided by each individual taxa remains largely unknown, though coccinellids seem to provide the most ACP control in Florida (Michaud 2004; Qureshi & Stansly 2007), a situation not seen in southern California. Work from Pakistan strongly suggests spiders are not important ACP predators in unsprayed citrus orchards (Vetter et al. 2013).
In California, syrphid (Allograpta species) and lacewing (Chrysoperla species) larvae have been identified as the dominant consumer species of ACP nymphs with combined predation rates inflicting 86% mortality (Kistner et al. 2016b). Numerous multi-predator studies have demonstrated a positive relationship between predator biodiversity and predation of insect herbivores in several different agricultural ecosystems (reviewed by Crowder & Jabbour 2014) and it is likely that natural enemy complementarity contributes to ACP biological control, especially in urban citrus where these studies were conducted.
Little is known about intraguild interactions amongst ACP natural enemies competing for ACP nymphs in California, but they are suspected to occur (Tena et al. 2013; Kistner & Hoddle 2016b). In Florida, generalist predators such as coccinellids can cause >95% mortality of ACP parasitized by T. radiata (Michaud 2004). Predation of parasitized ACP nymphs may be a key contributing factor to low parasitism estimates in the field (Michaud 2004; Qureshi & Stansly 2009).
Complex, intraguild interactions involving ants are also likely interfering with California’s biological control program for ACP. Direct and indirect parasitoid disruption by ants in agricultural systems is recognized as an important impediment to natural enemy efficacy (Delabie 2001; Ness & Bronstein 2004; Styrsky & Eubanks 2007; Helms 2013; Yao 2014). The positive, negative, or neutral impact of ant interference in a biological control program relies upon a complex mix of factors such as ant access to external sugar resources, the tending intensity by ants of pests that produce waste sugars (i.e., honeydew), ant aggression levels towards natural enemies attacking ant protected pests, and the length of parasitoid searching and oviposition behaviors which affects exposure times to foraging ants (Yoo & Holway 2011; Helms 2013; Sime & Daane 2014).
A key player in southern California citrus is the invasive Argentine ant, Linepithema humile (Mayr) (Hymenoptera: Formicidae) (Fig. 12[I13] ), a notorious pest of natural, urban, and agricultural systems. L. humile has thrived in southern California’s ideal Mediterranean climate for more than a century, with populations reaching exceptionally high densities in managed systems with food and irrigation runoff (Cook 1953; Holway et al. 2002). Both resources are abundant in citrus groves, where individual trees may receive nearly 1.5 million ant visits in a single day (Schall & Hoddle, unpublished data). A primary food source is the sugary waste excreted by phloem-feeding, honeydew-producing hemipterans (HPHs; e.g., mealybugs, scales, aphids, whiteflies, and ACP) that ants obtain through a mutually beneficial food-for-protection exchange (Fig. 12).
L. humile will readily form mutualisms with a wide variety of HPHs in the citrus agroecosystem, many of which are invasive economically damaging pests. L. humile-HPH mutualisms are problematic because tending ant workers aggressively defend their sugar-producing partners from predators and parasitoids (Choe & Rust 2006; Powell & Silverman 2010), which often reduces considerably the efficacy of biological control agents (Vega and Rust 2001; Helms 2013). Lower pressure from natural enemies in combination with ant provision of ‘sanitation services’ which reduce incidence of pathogen infection and honeydew drowning (Gullan 1997), ant dispersal of tended hosts to new foraging areas (Flanders 1951; Way 1963), and increased rates of phloem ingestion by tended HPHs and the resultant increased growth and reproductive rates (Yoo & Holway 2011), collectively facilitate population explosions of pest HPHs.
Subsequent HPH population growth and spreadwithin orchards provides an ever-increasing supply of honeydew for ants, fueling ant success and allowing pest HPHs and ants to invade new areas together (Davidson 1998; Silverman and Brightwell 2008; Rowles & Silverman 2009; Helms 2013). Increased HPH abundance can also expedite the spread of vectored plant diseases, growth of sooty molds from residual honeydew, and HPH-caused feeding damage such as leaf curling, foliar dieback, and stunted growth. Ultimately, the cost of ant-HPH interactions is a reduction in plant fitness and potentially economically significant crop loss if pest HPH and ants are not controlled with insecticides (Godfrey et al. 2002; Styrsky & Eubanks 2007). Thus, ant control is a critical constituent of IPM programs targeting ant-tended HPH pests, especially those that rely heavily upon biological control.
Despite our comprehensive understanding of the consequences of ant-HPH interactions, ant mutualisms with psyllids, including ACP, have received virtually no study (Delabie & Fernandez 2003). This is surprising, given the considerable biological control effort ongoing for ACP in the US (e.g., Florida [where ants are known to affect ACP biocontrol] and Texas), and California, in particular. What little information has been gathered, however, strongly suggests when ants form mutualisms with ACP, biological control of this pest declines.
In Florida, suppression of the dominant invasive ant species Pheidole megacephala (big-headed ant), Solenopsis invicta (red imported fire ant), Brachymyrmex obscurior, and Brachymyrmex patagonicus (rover ants) substantially improved ACP biocontrol by T. radiata (Navarette et al. 2013). In southern California, a survey of urban citrus gardens found more than half of ACP colonies were tended by L. humile and ACP parasitism by T. radiata was significantly higher in citrus lacking L. humile (Tena et al. 2013). To further investigate the implications of this relationship for ACP biocontrol, a series of replicated field experiments was conducted at urban and research citrus groves in Riverside, CA. The results of these trials indicated that when L. humile is controlled using Tanglefoot (a sticky physical barrier that excludes ants from HPH colonies) or liquid poison bait distributed in bait stations (i.e., 0.0001% thiamethoxam in a 25% sucrose solution at rate of 100mL/tree), ACP parasitism by T. radiata is 70 – 800% higher and generalist predators are, on average, 1 - 3.5 times more abundant when compared with ACP-infested branches on trees not being treated for ant infestations (Schall & Hoddle, unpublished data).
Antagonistic interactions between L. humile and natural enemies of ACP are likely responsible for the disparity in biocontrol between treatments. Field observations revealed that nearly all L. humile - T. radiata interactions on ant-infested branches resulted in parasitoid flight or death due to ant aggression. These ant-parasitoid conflicts frequently interrupted oviposition attempts by T. radiata, allowing ACP nymphs to escape parasitism. Taken together, these results suggest that L. humile disrupts natural enemies of ACP, thereby suppressing ACP biocontrol. Management of L. humile via a liquid baiting regime is a highly effective, sustainable strategy proven to improve biocontrol of ACP and likely that of several other ant-tended HPH pests such as the scales, aphids, whiteflies, and mealybugs that also infest citrus.
References
- Ammar, E. D., Shatters Jr, R. G., Lynch, C., and Hall, D. G. 2011. Detection and relative titer of Candidatus Liberibacter asiaticus in the salivary glands and alimentary canal of Diaphorina citri (Hemiptera: Psyllidae) vector of citrus huanglongbing disease. Annals of the Entomological Society of America. 104: 526-533.
- Arakawa, K., and K. Miyamoto. 2007. Flight ability of Asiatic citrus psyllid, Diaphorina citri Kuwayama (Homoptera; Psyllidae), measured by a flight mill. Research Bulletin of the Plant Protection Service. Japan 43: 23-26.
- Aubert, B. 1987. Le greening une maladie infetieuse des agrumes, d’origine bactereenne, transmise pardes homopteres. Strategie de lute developpee l’ile de la Reunion. Circumstances epidemiologiques en Afrique? Asie et modalities d’intervention. IRFA/CIAD-B.P. 180-97455 Saint Pierre Cedex.
- Barr, N.B., Hall, D.G., Weathersbee III, A.A., Nguyen, R., Stansly, P., Qureshi, J.A. and Flores, D., 2009. Comparison of laboratory colonies and field populations of Tamarixia radiata, an ectoparasitoid of the Asian citrus psyllid, using internal transcribed spacer and cytochrome oxidase subunit I DNA sequences. Journal of Economic Entomology. 102: 2325-2332.
- Bassanezi, R.B. and Bassanezi, R.C. 2008. An approach to model the impact of huanglongbing on citrus yield. In Proceedings of the International Research Conference on Huanglongbing. 301-304.
- Beattie GAC, Holford P, Haigh AM & Broadbent P (2009) On the origins of Citrus, huanglongbing, Diaphorina citri and Trioza erytreae. Proceedings of the International Research Conference on Huanglongbing, December 2008 (ed. by TR Gottwald & JH Graham), pp. 23–56. Plant Management Network, Orlando, FL, USA. Available here
- Bennet, R. 2016.Our #1 goal: controlling HLB. The ultimate and only objective. Citrograph. 7: 8-10.
- Bistline-East, A. and Hoddle, M.S., 2016. Biology of Psyllaphycus diaphorinae (Hymenoptera: Encyrtidae), a Hyperparasitoid of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae) and Tamarixia radiata (Hymenoptera: Eulophidae). Annals of the Entomological Society of America. 109: 22-28.
- Bistline-East, A., R. Pandey, M. Kececi, and M. S. Hoddle. 2015. Host range testing of Diaphorencyrtus aligarhensis(Hymenoptera: Encyrtidae) for use in classical biological control of Diaphorina citri (Hemiptera: Liviidae) in California. Journal of Economic Entomology. 108: 940–950.
- Boina,D.R., W.L.Meyer, E.O.Onagbola, and L.L. Stelinski. 2009. Quantifying dispersal of Diaphorina citri(Hemiptera:Psyllidae) by immunomarking and potential impact of unmanaged groves on commercial citrus management. Environmental Entomology 38: 1250-1258.
- Bové, Joseph, M. 2006. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. Journal of plant pathology. 88: 7-37.
- Brodersen, C., Narciso, C., Reed, M. and Etxeberria, E., 2014. Phloem production in Huanglongbing-affected citrus trees. Horticultural Science. 49: 59-64.
- Calflora, 2016. California Flora Database. Berkeley, CA. Link (accessed 29 January 2017).
- California Department of Food and Agriculture (CDFA), 2016. CDFA ACP/HLB Regulation and Quarantine Boundaries. Link (accessed 29 January 2017).
- Chen, X. and Stansly, P.A. 2014. Biology of Tamarixia radiata (Hymenoptera: Eulophidae), parasitoid of the citrus greening disease vector Diaphorina citri (Hemiptera: Psylloidea): a mini review. Florida Entomologist. 98:1404-1413.
- Chen, X., Bordini, G.P. and Stansly, P.A., 2016. Avoidance of parasitized hosts by female wasps of Tamarixia radiata(Hymenoptera: Eulophidae), parasitoid of Diaphorina citri (Hemiptera: Liviidae), vector of citrus greening disease. Florida Entomologist. 99: 311-313.
- Chien, C.C., Chu, Y.I. and Ku, S.C. 1991.Parasitic strategy, morphology and life history of Tamarixia radiata (Hymenoptera; Eulophidae).Chinese Journal of Entomology.11: 264-281.
- Chien, C.C., Chu, Y.I. and Ku, H.C. 1994. Oosorption and oviposition-regulating capability of the eulophid wasp, Tamarixia radiata, and its internal reproductive organs. Plant Protection Bulletin Taipei. 36: 19-30.
- Chien, C.C., Chu, Y.I. and Ku, S.C. 1995. Influences of host densities on the population increase of the eulophid wasp, Tamarixia radiata, and its host-killing ability. Plant Protection Bulletin Taipei. 37: 81-96.
- Choe, D., and Rust M. K. 2006. Agonistic behavior of argentine ants to scales and scale parasitoids and their cuticular extracts. Sociobiology. 48: 799-818.
- Chu, Y.I. and Chien, C.C. 1991. Utilization of natural enemies to control psyllid vectors transmitting citrus greening. Integrated control of plant virus diseases. Food and fertilizer technology center for the Asian and Pacific region, Taipei, Taiwan.135-145.
- Civerolo, E. 2015. Asian citrus psyllid and HLB detection in California. Citrograph 6: 8–9.
- Coletta-Filho, H.D., Daugherty, M.P., Ferreira, C. and Lopes, J.R., 2014. Temporal progression of ‘Candidatus Liberibacter asiaticus’ infection in citrus and acquisition efficiency by Diaphorina citri. Phytopathology. 104: 416-421.
- Cook, T. W. 1953. The Ants of California.Pacific Book, Palo Alto, CA. 462 pp.
- Crooks, J.A., Soulé, M.E. and Sandlund, O.T., 1999. Lag times in population explosions of invasive species: causes and implications. Invasive species and biodiversity management, pp.103-125.
- Crowder, D.W. and Jabbour, R., 2014. Relationships between biodiversity and biological control in agroecosystems: current status and future challenges. Biological Control. 75: 8-17.
- da Graça, J.V., 1991. Citrus greening disease. Annual review of phytopathology. 29: 109-136.
- da Graça, J.V. and Korsten, L., 2004. Citrus huanglongbing: Review, present status and future strategies. In Diseases of fruits and vegetables volume I (pp. 229-245). Springer Netherlands.
- Davidson, D. W. 1998. Resource discovery versus resource domination in ants: a functional mechanism for breaking the trade‐off. Ecological Entomology. 23: 484-490.
- Davidson, A.M., Jennions, M. and Nicotra, A.B., 2011. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta‐analysis. Ecology letters. 14: 419-431.
- Delabie, J. H. C. 2001. Trophobiosis between Formicidae and Hemiptera (Sternorrhyncha and Auchenorrhyncha): An overview. Neotropical Entomology. 30: 501-516.
- Delabie, J. H. C. and Fernández, F. 2003. Relaciones entre hormigas y homópteros (Hemiptera: Sternorrhyncha y Auchenorrhyncha). In Fernandez, F. [ed.], Introducción a las Hormigasde la Región Neotropical. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Bogotá, CO. 181-197.
- Etxeberria, E., Gonzalez, P., Achor, D. and Albrigo, G., 2009. Anatomical distribution of abnormally high levels of starch in HLB-affected Valencia orange trees. Physiological and Molecular Plant Pathology. 74: 76-83.
- European and Mediterranean Plant Protection Organization (EPPO), 2009. EPPO Reporting service: 2009/087 Successful control of insect vectors of citrus huanglongbing in the islands of Reunion, Mauritius and Guadeloupe pp.4.
- Flanders, S. E. 1951. The role of the ant in the biological control of homopterous insects. The Canadian Entomologist. 83: 93-98.
- Folgarait, P. J. 1998. Ant biodiversity and its relationship to ecosystem functioning: a review. Biodiversity and Conservation. 7: 1221-1244.
- Fung, Y.C. and Chen, C.N., 2006. Effects of temperature and host plant on population parameters of the citrus psyllid (Diaphorina citri Kuwayama). Formosan Entomologist. 26: 109-123.
- Gandhi, K.J. and Herms, D.A., 2010. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biological Invasions. 12: 389-405.
- Giliomee, J. H. 1986. Seed dispersal by ants in the Cape flora threatened by Iridomyrmex humilis (Hymenoptera: Formicidae). Entomologia Generalis. 11: 217-219.
- Godfrey, K. E. 2002. Mealybugs in California vineyards. UCANR Publications.Vol. 21612.
- Goldson, S.L., Rowarth, J.S. and Caradus, J.R., 2005. The impact of invasive invertebrate pests in pastoral agriculture: a review. New Zealand Journal of Agricultural Research. 48: 401-415.
- González-Hernández, H., Johnson, M. W., and Reimer, N. J. 1999.Impact of Pheidole megacephala (F.) (Hymenoptera: Formicidae) on the biological control of Dysmicoccus brevipes(Cockerell) (Homoptera: Pseudococcidae). Biological Control. 15: 145-152.
- Gottwald, T. R. 2007. Citrus canker and citrus huanglongbing, two exotic bacterial diseases threatening the citrus industries of the Western Hemisphere. Outlooks on Pest Management. 18: 274-279.
- Gottwald, T.R. 2010.Current epidemiological understanding of citrus huanglongbing. Annual Review of Phytopathology. 48: 119-139.
- Grafton-Cardwell, E.E., 2005. Asian citrus psyllid. UCANR Publications.
- Grafton-Cardwell, E.E., Stelinski, L.L. and Stansly, P.A., 2013. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annual Review of Entomology. 58: 413-432.
- Graham, J.H., Johnson, E.G., Gottwald, T.R. and Irey, M.S., 2013. Presymptomatic Fibrous Root Decline in Citrus Trees Caused by Huanglongbing and Potential Interaction with Phytophthora spp. Plant Disease. 97: 1195-1199.
- Gullan P. J., 1997. Relationships with ants. In: Ben-Dov, Y. and Hodgson, C. J. (Eds.) World crop pests: Soft scale insects, their biology, natural enemies and control. Elsevier, New York. pp. 351-373.
- Halbert, S.E. and Manjunath, K.L. 2004. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: a literature review and assessment of risk in Florida. Florida Entomologist. 87: 330-353.
- Halbert S.E., Manjunath K.L., Ramadugu C., Brodie M.W., Webb S.E. & Lee R.F., 2010. Trailers transporting oranges to processing plants move Asian citrus psyllids. Florida Entomologist 93: 33–38.
- Hall, D. G., and Gottwald, T. R. 2011. Pest management practices aimed at curtailing citrus huanglongbing disease. Outlooks on Pest Management. 22: 189-192.
- Hall, D. G., and Nguyen, R. 2010. Toxicity of pesticides to Tamarixia radiata, a parasitoid of the Asian citrus psyllid. BioControl. 55: 601-611.
- Hall, D.G., Hentz, M.G. and Adair Jr, R.C., 2008. Population ecology and phenology of Diaphorina citri (Hemiptera: Psyllidae) in two Florida citrus groves. Environmental Entomology. 37: 914-924.
- Hall, D.G., Hentz, M.G., Meyer, J.M. & Boucias, D.G. 2012. Observations on the entomopathogenic fungus Hirsutella citriformis attacking adult Diaphorina citri (Hemiptera: Psyllidae) in a managed citrus grove. BioControl 57: 663–675.
- Hall, D. G., Richardson, M. L., Ammar, E. D., and Halbert, S. E. 2013. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomologia Experimentalis etApplicata. 146: 207-223.
- Hall, D.G., Wenninger, E.J. & Hentz, M.G. 2011. Temperature studies with the Asian citrus psyllid, Diaphorina citriKuwayama: cold hardiness and temperature thresholds for oviposition. Journal of Insect Science 11: 1–15.
- Helms, K. R. 2013. Mutualisms between ants (Hymenoptera: Formicidae) and honeydew-producing insects: Are they important in ant invasions? Myrmecological News.18: 61-71.
- Hoddle, M.S. and Hoddle, C.D., 2013. Classical biological control of Asian citrus psyllid with Tamarixia radiata in urban Southern California. Citrograph, 4: 52-58.
- Hoddle, M. S., C. D. Hoddle, S. V. Triapitsyn, S. Z. Khan, and M. J. Arif. 2014. How many primary parasitoid species attack nymphs of Diaphorina citri (Hemiptera: Liviidae) in Punjab, Pakistan? Florida Entomologist. 97: 1825–1828.
- Hoddle, M.S. 2004. Restoring balance: using exotic species to control invasive exotic species. Conservation Biology. 18: 38-49.
- Hoddle, M.S. 2012. Foreign exploration for natural enemies of Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae), in the Punjab of Pakistan for use in a classical biological control program in California, USA. Pakistan Entomologist. 34: 1-5.
- Hodges, A., and Spreen, T. H. 2012. Economic Impacts of Citrus Greening (HLB) in Florida, 2006/07–2010/11. Food and Resource Economics Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, Gainesville.1-6.
- Hoddle, M.S. and Pandey, R. 2014. Host range testing of Tamarixia radiata (Hymenoptera: Eulophidae) sourced from the Punjab of Pakistan for classical biological control of Diaphorina citri (Hemiptera: Liviidae: Euphyllurinae: Diaphorinini) in California. Journal of Economic Entomology. 107: 125-136.
- Holway, D. A., Lach, L., Suarez, A. V., Tsutsui, N. D., and Case, T. J. 2002. The causes and consequences of ant invasions. Annual Review of Ecology and Systematics. 33: 181-233.
- Hopper, K.R., Roush, R.T. and Powell, W., 1993. Management of genetics of biological-control introductions. Annual review of entomology. 38: 27-51.
- Hornbaker, V., and L. Kumagai. 2016. HLB detections in San Gabriel. Where Are We Now? Citrograph 7: 24–27.
- Huang, C.H.M., Y. Tsai, and C. L.Wang. 1984. Transmission of citrus likubin by a psyllid, Diaphorina citri. Journal of Agricultural Research. China 33: 65-72.
- Hung, T. H., S. C. Hung, C. N. Chen, M. S. Hsu, and H. J. Su. 2004. Detection by PCR of Candidatus Liberibacter asiaticus, the bacterium causing citrus huanglongbing in vector psyllids: application to the study of vector-pathogen relationships. Plant Pathology. 53: 96-102.
- Hussain, M. A., and D. Nath. 1927. The citrus psylla (Diaphorina citri Kuw.) (Psyllidae: Homoptera), pp. 5–27. Indian Department of Agriculture Memoirs Entomological Series, vol. 10. Government of India Central Publication Branch.
- Ikpechukwu, C.O., C.A. Sims, M.D. Danyluk, T.M. Spann, and R.M. Goodrich. 2011. Effect of fruit size and huanglongbing disease on orange juice attributes. Proceedings of the Florida State Horticultural Society. 124:202–206
- Inoue, H., Ohnishi, J., Ito, T., Tomimura, K., Miyata, S., Iwanami, T., and Ashihara, W. 2009. Enhanced proliferation and efficient transmission of Candidatus Liberibacter asiaticus by adult Diaphorina citri after acquisition feeding in the nymphal stage. Annals of Applied Biology. 155: 29-36.
- Jervis, M.A., Heimpel, G.E., Ferns, P.N., Harvey, J.A. and Kidd, N.A. 2001. Life‐history strategies in parasitoid wasps: a comparative analysis of ‘ovigeny’. Journal of Animal Ecology. 70: 442-458.
- Johnson, E.G., Wu, J., Bright, D.B. and Graham, J.H., 2014. Association of ‘Candidatus Liberibacter asiaticus’ root infection, but not phloem plugging with root loss on huanglongbing‐affected trees prior to appearance of foliar symptoms. Plant pathology. 63: 290-298.
- Juan-Blasco, M., Qureshi, J.A., Urbaneja, A. and Stansly, P.A., 2012. Predatory mite, Amblyseius swirskii (Acari: Phytoseiidae), for biological control of Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Florida Entomologist. 95: 543-551.
- Keane, R.M. and Crawley, M.J., 2002. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution. 17: 164-170.
- Keremane, M. L., C. Ramadugu, E. Rodriguez, R. Kubota, S. Shibata, D. G. Hall, M. L. Roose, D. Jenkins, and R. F. Lee. 2015. A rapid field detection system for citrus huanglongbing associated ‘Candidatus Liberibacter asiaticus’ from the psyllid vector, Diaphorina citri Kuwayama and its implications in disease management. Crop Protection. 68: 41–48.
- Khan, S.Z., Arif, M.J., Hoddle, C.D. and Hoddle, M.S., 2014. Phenology of Asian citrus psyllid (Hemiptera: Liviidae) and associated parasitoids on two species of citrus, kinnow mandarin and sweet orange, in Punjab Pakistan. Environmental entomology. 43: 1145-1156.
- Kistner, E.J., Amrich, R., Castillo, M., Strode, V. and Hoddle, M.S., 2016. Phenology of Asian Citrus Psyllid (Hemiptera: Liviidae), With Special Reference to Biological Control by Tamarixia radiata, in the Residential Landscape of Southern California. Journal of Economic Entomology. 0: 1-11.
- Kistner, E.J., Melhem, N., Carpenter, E., Castillo, M. and Hoddle, M.S., 2016. Abiotic and Biotic Mortality Factors Affecting Asian Citrus Psyllid (Hemiptera: Liviidae) Demographics in Southern California. Annals of the Entomological Society of America. p.saw053.
- Kumagai, L. B., C. S. LeVesque, C. L. Blomquist, K. Madishetty, Y. Guo, P. W. Woods, S. Rooney-Latham, J. Rascoe, T. Gallindo, D. Schnabel, et al. 2013. First report of Candidatus Liberibacter asiaticus associated with citrus huanglongbing in California. Plant Disease. 97: 283.
- Leavitt, R., 2012. Huanglongbing confirmed in California. Citrograph, 3: 8-9.
- Lee, J.A., Halbert, S.E., Dawson, W.O., Robertson, C.J., Keesling, J.E. and Singer, B.H., 2015. Asymptomatic spread of huanglongbing and implications for disease control. Proceedings of the National Academy of Sciences. 112: 7605-7610.
- Lewis-Rosenblum, H., Martini, X., Tiwari, S. and Stelinski, L.L. 2015.Seasonal movement patterns and long-range dispersal of Asian citrus psyllid in Florida citrus. Journal of Economic Entomology. 108: 3-10.
- Liu, Y.H. and Tsai, J.H., 2000. Effects of temperature on biology and life table parameters of the Asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae). Annals of applied biology. 137: 201-206.
- Lodge, D.M., Williams, S., MacIsaac, H.J., Hayes, K.R., Leung, B., Reichard, S., Mack, R.N., Moyle, P.B., Smith, M., Andow, D.A. and Carlton, J.T., 2006. Biological invasions: recommendations for US policy and management. Ecological applications. 16: 2035-2054.
- Mann, R.S., Ali, J.G., Hermann, S.L., Tiwari, S., Pelz-Stelinski, K.S., Alborn, H.T. and Stelinski, L.L., 2012. Induced release of a plant-defense volatile ‘deceptively’attracts insect vectors to plants infected with a bacterial pathogen. PLoS Pathogens. 8: p.e1002610.
- Martini, X., Hoyte, A. and Stelinski, L.L., 2014. Abdominal color of the Asian citrus psyllid (Hemiptera: Liviidae) is associated with flight capabilities. Annals of the Entomological Society of America. 107: 842-847.
- McFarland, C.D. and Hoy, M.A. 2001. Survival of Diaphorina citri (Homoptera: Psyllidae), and its two parasitoids, Tamarixiaradiata (Hymenoptera: Eulophidae) and Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae), under different relative humidities and temperature regimes. Florida Entomologist. 227-233.
- Messing, R.H. and Wright, M.G., 2006. Biological control of invasive species: solution or pollution?. Frontiers in Ecology and the Environment. 4: 132-140.
- Michaud, J. 2004. Natural mortality of Asian citrus psyllid (Homoptera: Psyllidae) in central Florida. Biological Control 29: 72-78.
- Michaud, J.P. and Olsen, L.E., 2004. Suitability of Asian citrus psyllid, Diaphorina citri, as prey for ladybeetles. BioControl. 49: 417-431.
- Mohan, G. 2015. Deadly citrus disease found in San Gabriel Valley. Los Angeles Times Online Archive. Link (accessed 29 January 2017).
- Monzó, C., Arevalo, H.A., Jones, M.M., Vanaclocha, P., Croxton, S.D., Qureshi, J.A. and Stansly, P.A., 2015. Sampling methods for detection and monitoring of the Asian citrus psyllid (Hemiptera: Psyllidae). Environmental entomology. 44: 780-788.
- Nava, D.E., Torres, M.L.G., Rodrigues, M.D.L., Bento, J.M.S. and Parra, J.R.P., 2007. Biology of Diaphorina citri (Hem., Psyllidae) on different hosts and at different temperatures. Journal of Applied Entomology. 131: 709-715.
- Navarrete, B., McAuslane, H., Deyrup, M., and Peña, J. E. 2013. Ants (Hymenoptera: Formicidae) associated with Diaphorina citri (Hemiptera: Liviidae) and their role in its biological control. Florida Entomologist. 96: 590-597.
- Ness, J. H., and Bronstein, J. L. 2004.The effects of invasive ants on prospective ant mutualists. Biological Invasions. 6: 445-461.
- Pelz-Stelinski, K.S. and Killiny, N., 2016. Better together: Association with ‘Candidatus Liberibacter asiaticus’ increases the reproductive fitness of its insect vector, Diaphorina citri (Hemiptera: Liviidae). Annals of the Entomological Society of America. 109: 371-376.
- Pelz-Stelinski, K. S., Brlansky, R. H., Ebert, T. A., and Rogers, M. E. 2010. Transmission parameters for CandidatusLiberibacterasiaticus by Asian citrus psyllid (Hemiptera: Psyllidae). Journal of Economic Entomology. 103: 1531-1541.
- Pérez-Otero R., Mansilla J.P., del Estal P., 2015. Detecci on de la psila Africana de los cıtricos, Trioza erytreae (Del Guercio, 1918) (Hemiptera: Psylloidea: Triozidae), en la Penınsula Iberica. Arq Entomol 13: 191–122.
- Pourreza, A., Lee, W.S., Etxeberria, E. and Banerjee, A., 2015. An evaluation of a vision-based sensor performance in Huanglongbing disease identification. Biosystems Engineering. 130: 13-22.
- Powell, B.E. and Silverman, J. 2010. Impact of Linepithe mahumile and Tapinoma sessile (Hymenoptera: Formicidae) on three natural enemies of Aphis gossypii (Hemiptera: Aphididae). Biological Control. 54: 285-291.
- Purcell, A. H. 1982. Insect vector relationships with procaryotic plant pathogens Annual Reviews of Phytopathology. 20: 397-417.
- Qureshi, J.A. and Stansly, P.A. 2007. Integrated approaches for managing the Asian citrus psyllid Diaphorina citri(Homoptera: Psyllidae) in Florida. In Proc. Florida State Horticultural Society. 120: 110-115.
- Qureshi, J. A. and Stansly, P. 2009. Exclusion techniques reveal significant biotic mortality suffered by Asian citrus psyllid Diaphorina citri (Hemiptera: Psyllidae) populations in Florida citrus. Biological Control. 50: 129-136.
- Richards, T. J., D. W. Shanafelt, and E. P. Fenichel. 2014. Foreclosures and invasive insect spread: the case of Asian citrus psyllid. American Journal of Agricultural Economics. 96: 615–630.
- Richardson M.L. & Hall D.G., 2012. Resistance of Poncirus and Citrus x Poncirus germplasm to the Asian citrus psyllid. Crop Science. doi:10.2135/cropsci2012.02.0091.
- Rohrig, E.A., Hall, D.G., Qureshi, J.A. and Stansly, P.A., 2012. Field release in Florida of Diaphorencyrtus aligarhensis(Hymenoptera: Encyrtidae), an endoparasitoid of Diaphorina citri (Homoptera: Psyllidae), from mainland China. Florida Entomologist. 95: 479-481.
- Rohrig, E., Shirk, P.D., Hall, D.G. and Stansly, P.A., 2011. Larval development of Diaphorencyrtus aligarhensis(Hymenoptera: Encyrtidae), an endoparasitoid of Diaphorina citri (Hemiptera: Psyllidae). Annals of the Entomological Society of America. 104: 50-58.
- Roistacher, C. N. 1991. Greening, pp. 35-45. In Techniques for biological detection of specific citrus graft transmissible diseases. Food and Agricultural Organization of the United Nations, Rome, Italy.
- Roush, R.T. and Hopper, K.R., 1995. Use of single family lines to preserve genetic variation in laboratory colonies. Annals of the Entomological Society of America. 88: 713-717.
- Rowles, A. D., and Silverman, J. 2009. Carbohydrate supply limits invasion of natural communities by Argentine ants. Oecologia. 161: 161-171.
- Sakai, A.K., Allendorf, F.W., Holt, J.S., Lodge, D.M., Molofsky, J., With, K.A., Baughman, S., Cabin, R.J., Cohen, J.E., Ellstrand, N.C. and McCauley, D.E., 2001. The population biology of invasive species. Annual review of ecology and systematic. 32: 305-332.
- Sakamaki, Y., 2005. Possible migration of the Asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae) between and within islands. Occasional Papers of the Kagoshima University Research Center. 42: 121-125.
- Silverman, J., and Brightwell, R. J. 2008. The Argentine ant: challenges in managing an invasive unicolonial pest. Annual Review of Entomology. 53: 231-252.
- Sime, K.R. and Daane, K.M. 2014. A Comparison of Two Parasitoids (Hymenoptera: Encyrtidae) of the Vine Mealybug: Rapid, Non-Discriminatory Oviposition Is Favored When Ants Tend the Host. Environmental Entomology. 43: 995-1002.
- Skelley, L. H., and M. A. Hoy. 2004. A synchronous rearing method for the Asian citrus psyllid and its parasitoids in quarantine. Biological Control. 29: 14-23.
- Spann, T, M., Schumann, A. W., Rouse, B., and Ebel, B. 2011.Foliar nutrition for HLB.Citrus Industry. 92: 6-10.
- Spreen, T.H., Baldwin, J.P. and Futch, S.H., 2014. An economic assessment of the impact of Huanglongbing on citrus tree plantings in Florida. Horticultural Science. 49: 1052-1055.
- Strong, D.R. and Pemberton, R.W., 2000. Biological control of invading species--risk and reform. Science. 288: 1969-1970.
- Styrsky, J.D. and Eubanks, M.D. 2007.Ecological consequences of interactions between ants and honeydew-producing insects. Proceedings of the Royal Society of London B: Biological Sciences. 274: 151-164.
- Tang, Y.Q. and Wu, M.X., 1991. Interspecific host discrimination between two primary parasites of Asian citrus psyllid Diaphorina citri Kuwayama. Pp. 99-104 in C. Ke, and S.B. Osman, es. Proc. 6th Intern. Asia Pacific Workshop Integrated Citrus Health Management. Kuala Lumpur.
- Teixeira D.C., Saillard C., Couture C., Martins E.C.,Wulff N.A., et al. 2008. Distribution and quantification of Candidatus Liberibacter americanus, agent of huanglongbing disease of citrus in Sao Paulo State, Brasil, in leaves of an affected sweet orange tree as determined by PCR. Molecular and Cellular Probes.22:139–50
- Tena, A., Hoddle, C. D., and Hoddle, M. S. 2013. Competition between honeydew producers in an ant–hemipteran interaction may enhance biological control of an invasive pest. Bulletin of entomological research. 103: 714-723.
- Tiwari, S., Mann, R. S., Rogers, M. E., and Stelinski, L. L. 2011.Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest management science. 67: 1258-1268.
- Tiwari, S., N. Killiny, R. S. Mann, E. J. Wenninger, and L. L. Stelinski. 2013. Abdominal color of the Asian citrus psyllid, Diaphorina citri, is associated with susceptibility to various insecticides. Pest Management Science. 69: 535-541.
- Tsai, J. H., and Y. H. Liu. 2000. Biology of Diaphorina citri (Homoptera : Psyllidae) on four host plants. Journal of Economic Entomology. 93: 1721-1725.
- Ukuda-Hosokawa, R., Y. Sadoyama, M. Kishaba, T. Kuriwada, H. Anbutsu, and T. Fukatsu. 2015. Infection density dynamics of the citrus greening bacterium “Candidatus Liberibacter asiaticus” in filed populations of the psyllid Diaphorina citri and its relevance to the efficiency of pathogen transmission to citrus plants. Applied Environmental Microbiology 81: 3728–3736
- United States Department of Agriculture Citrus Health Response Plan (USDA CHRP), 2016. USDA Efforts to Combat Huanglongbing (HLB). Link (accessed 29 January 2017).
- United States Department of Agriculture National Agricultural Statistics Services (USDA NASS), 2016a. Citrus Production Forecast. Link (accessed 29 January 2017).
- United States Department of Agriculture National Agricultural Statistics Services (USDA NASS), 2016b. Citrus Production Report. Link (accessed 29 January 2017).
- Vankosky M.A. and M.S. Hoddle. 2016. Biological control of ACP using Diaphorencyrtus aligarhensis. Citrograph. 7: 68-72.
- Vega, S.J. and Rust, M.K. 2001.The Argentine Ant- A Significant Invasive Species in Agricultural, Urban and Natural Environments.Sociobiology. 37: 3-26.
- Vetter, R. S., S. Z. Khan, M. J. Arif, C. Hoddle, and M. S. Hoddle. 2013. Spiders (Araneae) surveyed from unsprayed citrus orchards in Faisalabad, Pakistan and their potential as biological control agents of Diaphornia citri (Hempitera: Liviidae). Pakistan Entomologist. 35: 61–69.
- Wang, Z., Yin, Y., Hu, H., Yuan, Q., Peng, G. and Xia, Y., 2006. Development and application of molecular‐based diagnosis for ‘Candidatus Liberibacter asiaticus’, the causal pathogen of citrus huanglongbing. Plant Pathology. 55: 630-638.
- Waterston, J., 1922. On the chalcidoid parasites of psyllids (Hemiptera, Homoptera). Bulletin of Entomological Research. 13: 41-58.
- Way, M. J. 1963: Mutualism between ants and honeydew producing Homoptera. Annual Review of Entomology. 8: 307-344.
- Wenninger, E., and D. G. Hall. 2007. Daily mating and age at reproductive maturity in Diaphornia citri (Hemiptera: Psyllidae). Florida Entomologist. 90: 715–722.
- Wenninger, E. J., and D. G. Hall. 2008. Daily and seasonal patterns in abdominal color in Diaphorina citri(Hemiptera:Psyllidae). Annals of the Entomological Society of America. 101: 585-592.
- Wenninger, E. J., L. L. Stelinski, and D. G. Hall. 2009. Relationships between adult abdominal color and reproductive potential in Diaphorina citri (Hemiptera: Psyllidae). Annals of the Entomological Society of America. 102: 476-483.
- Wetterich, C.B., de Oliveira Neves, R.F., Belasque, J. and Marcassa, L.G., 2016. Detection of citrus canker and Huanglongbing using fluorescence imaging spectroscopy and support vector machine technique. Applied optics. 55: 400-407.
- Xu CF, Xia YH, Li KB & Ke C (1988) Further study of the transmission of citrus huanglongbing by a psyllid, Diaphorina citriKuwayama. Proceedings of the 10th Conference of the International Organization of Citrus Virologists, 17–21 November 1986, Valencia, Spain (ed. by LW Timmer, SM Garnsey & L Navarro), pp. 243–248. International Organization of Citrus Virologists, University of California, Riverside, CA, USA.
- Yang, Y., Huang, M., C. Beattie, G.A., Xia, Y., Ouyang, G. and Xiong, J., 2006. Distribution, biology, ecology and control of the psyllid Diaphorina citri Kuwayama, a major pest of citrus: a status report for China. International Journal of Pest Management. 52: 343-352.
- Yao, I. 2014. Costs and constraints in aphid-ant mutualism.Ecological research. 29: 383-391.
- Yoo, H.J.S. and Holway, D.A. 2011. Context‐dependence in an ant–aphid mutualism: direct effects of tending intensity on aphid performance. Ecological Entomology. 36: 450-458.