Biological Control of Icerya purchasi with Rodolia cardinalis in the Galapagos
Prepared by Mark Hoddle, Extension Specialist and Director of Center for Invasive Species Research
mark.hoddle@ucr.edu
Introduction |
Invasive species are the principal threat to the unique biota of the Galapagos Islands. Every month, at least six cargo boats and 80 commercial flights transport humans, cargo, and luggage to support an annual tourist trade exceeding 100,000 visitors per year and a resident population of 21,000 people. Introductions of exotic insects have increased exponentially and this is a direct result of increasing tourism and population growth (~6% per year) in the islands. Alien or exotic insects now constitute 23% of the Galapagos insect fauna. The six most damaging of these resident invaders are two species of fire ant, two species of wasp, an ectoparasitic fly, and a sap sucking bug known as the cottony cushion scale.
Cottony cushion scale, Icerya purchasi Maskell (Hemiptera: Coccoidea: Monophlebidae: Iceryini), an exotic plant pest in the Galapagos Islands, has been the target of a classical biological control program with the predatory beetle, Rodolia cardinalis (Mulsant) (Coleoptera: Coccinellidae). Icerya purchasi (aka el pulgon) is a cosmopolitan plant pest that is native to Australia and possibly New Zealand and is a known pest on over 200 different plant species. High density I. purchasi populations can be particularly damaging to native plants with restricted ranges. The debilitating effect that I. purchasi can have on rare native or endemic plants may also indirectly affect native vertebrates and invertebrates that are dependent on those plants for food and shelter. Consequently, it was anticipated that I. purchasi would attack plants in agricultural and urban areas in the Galapagos Islands following its successful establishment in 1982, and subsequent field observations indicated that it was likely to become an important pest in natural areas by attacking native and endemic plants with high conservation value.
Over the last 120 years, I. purchasi populations have been suppressed in numerous countries with R. cardinalis. One reason for this high level of success is due to the fact that R. cardinalis is a specialist predator that has a very restricted prey range, one that is probably limited to the family Monophlebidae, and possibly the tribe Iceryini. Strong prey fidelity by R. cardinalis has two major advantages in a classical biological control program: (1) high safety results because little threat is posed to non-target species because they are unsuitable food sources, and (2) high levels of population suppression occur because tight ecological and biological linkages ensure that maximum feeding and reproductive pressure are maintained on the target because R. cardinalis is unable to attack and breed on other prey species.
Biology of Icerya purchasi |
Reproduction. Icerya purchasi is a functional hermaphrodite, that is, an adult has both male and female reproductive organs and is capable of self-fertilization which eliminates the need to mate with other individuals of the same species. The first instar crawler has paired gonads comprised of female and male parts. In the second instar larva, the reproductive cells are divided into two groups, large diploid cells that develop into female gametes, and smaller haploid cells that develop into male gametes. In the third instar larva, the female part of the gonad is composed of young ovarioles, and the male part consists of spermatids (these later develop into spermatozoa) contained within a testis follicle. When the third instar larva molts into the adult stage, the ovarioles (egg containing tubes) and testicular tubules (containing sperm) are connected to a common duct that enables self fertilization of eggs. In this system, hermaphrodites always produce hermaphrodites only, and very rarely are winged males produced.
Sexual differentiation, should it occur, happens in the second instar. At this stage, males can be identified by their more slender body form and higher setal densities. Once the third instar is reached, males pupate in a thin silk cocoon either in a protective crevice on the host plant, or it may drop to the ground to pupate in the soil or beneath fallen leaves. The winged males that emerge from cocoons have dark red bodies. Hermaphrodites that mate with males may produce hermaphrodite only offspring, or hermaphrodites and a very small proportion of males. Females that must mate with males to produce offspring are unknown. Parthenogenetic development, the ability to produce offspring from unfertilized eggs, is not known to occur in I. purchasi.
Life cycle. Immature I. purchasi develop within the protective white fluted or grooved wax sac that grows behind the yellowy-orangey-brown colored body of the adult. The egg sac (or ovisac) can be 2-2.5 times the length of the body which can give an adult I. purchasi a length of 10-15 mm. Fertilized eggs are bright red and are discharged from the body into the ovisac. Typically, an ovisac may contain 600-800 eggs, but over 1000 eggs have sometimes been recorded. Dissection and inspection of the ovisac contents under a microscope reveals that there is an obvious age gradient of sac contents. Newly deposited eggs are closest to the body of the adult, mature eggs are in the center of the sac, and at the end of the sac most distant from the adult body, young crawlers can be found. Crawlers emerge from the ovisac as it splits, they have legs and are able to disperse from the adult either by walking, by being blown by the wind, or hitch-hiking on other organisms (e.g., clothing of people brushing against infested plants). Consequently, when heavy infestations of I. purchasi develop on plants it is typical to find all life stages present (reproductive adults, immature larvae, and crawlers).
I. purchasi has 5 developmental stages: the egg, the first instar or crawler stage (is red in color, have thin brown legs, and 6-segmented antennae) which disperses, the second (yellow in color) and third instars (reddish-brown in color with 9-segmented antennae) are relatively sedantary feeding stages, and the adult, the fourth and final instar has 11-segmented antennae and the characteristic ovisac begins to grow as soon egg laying commences and ovisac growth continues as long as oviposition lasts
Feeding and honey dew production. Icerya purchasi larvae and adults have "piercing-sucking" mouthparts that are inserted into suitable plant tissue. This pest causes damage mainly to leaves, stems, and branches of host plants by sucking sap and removing nutrients. I. purchasi prefers to feed on the undersides of leaves because the cuticle and epidermis is thinner here and it is easier for the mouthparts to reach the phloem (most preferred feeding site), secretory canals (intermediate preference), and the xylem (least preffered feeding site). This insect can feed on the woody parts of plants, but its ability to reach preferred feeding sites is less successful probably because the sclerenchyma surrounding the cortex makes it difficult to insert mouthparts and access the phloem. When the mouthparts have been inserted into a suitable feeding site, I. purchasi secretes a transparent sheath around its feeding stylets. It is possible that this sheath froms a canal that allows the mouthparts to be moved back and forth without breaking.
Feeding I. purchasi excrete sticky honey dew. This sugary substance is a waste product produced from feeding on phloem extracted from host plants. Honey dew is a highly attractive food source for ants, and also some species of natural enemies, including R. cardinalis. Depending on the type of plant I. purchasi is feeding on, the quality of honey dew as food source for natural enemies can be vary. For example, when I. purchasi feeds on toxic plants (e.g., Spartium junceum or Erythrina corallodendrum) the honey dew that is excreted is high in alkaloids. The developmental times for larvae and pupae of R. cardinalis are siginficantly higher when they feed on honey dew high in alkaloids when compared to conspecifics that are feeding on I. purchasi honey dew produced from non-alkaloid plant hosts like citrus. Consequently, pest populations can be higher on plants that provide some level of chemical defense against natural enemies.
Biological Control of Icerya purchasi |
Icerya purchasi was first discovered in the Galapagos Islands in 1982. It was likely imported accidentally on plant material from mainland South America. Small crawlers were readily dispersed by the wind and the inter-island movement of plants by humans probably assisted the spread of I. purchasi to at least 15 of the 18 main islands that form the Galapagos archipelago. Populations of I. purchasi increased to damaging levels because this pest was not being exploited by resident natural enemies and its population growth was solely limited by the availability of suitable host plants. Surveys across islands indicated that high density populations of I. purchasi were restricting plant growth and possibly killing hosts of at least 62 native or endemic species, 16 of which were listed as threatened in the IUCN Red List of Threatened Species, from which a further six were classified as Endangered or Critically Endangered.
The Charles Darwin Foundation and the Galapagos National Park Service considered I. purchasi a serious conservation threat. A Technical Advisory Committee assembled to address this invasion problem in 1996 concluded that this pest could not be mitigated with insecticides, and given the urgency of the problem biological control should be considered. Rodolia cardinalis was identified by the Technical Advisory Committee as the most suitable biological control agent for use against I. purchasi in the Galapagos because this natural enemy had controlled I. purchasi in other parts of the world and the majority of available evidence suggested that it also had a very restricted prey range indicating it probably would not threaten other insects, especially native and endemic species.
However, before the first biological control program to be launched in the Galapagos could be initiated, the safety of R. cardinalis had to be determined before this natural enemy could be released into the environment. The deliberate importation and release of an exotic species that would likely be invasive and have the capacity to spread unassisted throughout the Galapagos archipelago was controversial. To address legitimate concerns about the potential threat R. cardinalis posed to non-target insect species, especially native and endemic species, feeding experiments were conducted in a secure quarantine facility at the Charles Darwin Research Station in Puerto Ayora, Santa Cruz.
In March 1999, R. cardinalis was collected by CSIRO Entomology in Brisbane, Australia and imported into the quarantine facility that had been specially built for this project. In quarantine, 16 insect species from three different orders (Hemiptera, Coleoptera, and Neuroptera) and nine familes (Ortheziidae, Monophlebidae, Pseudococcidae, Eriococcidae, Coccidae, Diaspididae, Aphididae, Coccinellidae, and Chrysopidae) were tested for their suitability as prey for larval and adult R. cardinalis. The native species tested included those that were presumed to have high risk of being preyed upon by R. cardinalis.
Upon completion of safety testing experiments in Quarantine it was concluded that R. cardinalislarvae and adults posed no significant threat to native insects in the Galapagos because larvae were unable to complete development on non-target prey species, and adults exhibited a very narrow prey range also, preferring to attack and feed on the target, I. purchasi. Additionally, R. cardinalis posed no measurable threat to other predacious insects on the Galapagos because competition for prey was not likely occur because R. cardinalis has a strong predilection for I. purchasi, a food source not being used to any significant extent by any other organism in the Galapagos.
In 2002, permission was granted for the release of R. cardinalis from quarantine and mass rearing, distribution, and establishment efforts began. Priority areas for introductions were identified and 11 islands received releases of R. cardinalis (Santa Cruz, San Cristobal, Floreana, Isabela, Santiago, Fernandina, Marchena, Pinta, Pinzon, Rabida, and Genovesa). The beetle established easily because of the abundance of I. purchasi and R. cardinalis brought about swift reductions in pest densities. Exclusion experiments using sleeve cages and post-introduction monitoring conducted on heavily infested white mangrove stands (Laguncularia racemosa L.) in Puerto Ayora on Santa Cruz Island showed a rapid decrease in I. purchasi populations within 12 weeks of liberating R. cardinalis.
The biological control program against I. purchasi with R. cardinalis, now at seven years post-release of the natural enemy, is mature enough to be evaluated for its longer term impacts. In October 2009, a two year evaluation project for I. purchasi biological control with R. cardinaliswas initiated. For three months (Oct. – Dec. 2009) survey and experimental work was conducted by M.S. Hoddle, C.D., Hoddle (both University of California, Riverside), and R.G. Van Driesche (University of Massachusetts, Amherst) in cooperation with the Charles Darwin Foundation, the Galapagos National Park Service, and SICGAL (The Galapagos Quarantine and Inspection Service). Research is ongoing and is being conducted on Santa Cruz Island by Claudio Crespo Ramirez, a volunteer with the Charles Darwin Research Station, and by Jose Loayza (SICGAL) on the island of San Cristobal.
Preliminary conclusions from completed evaluations on the biological control of Icerya purchasi with Rodolia cardinalisin the Galapagos. While work is still ongoing, data collected in October-December, 2009 suggest that: (1) the introduced predator Rodolia cardinalis has survived and spread and is now widely present in many areas and habitats, and it has even been found attacking I. purchasi on islands it was not originally released on (i.e., Isla Champion). (2) The pest (Icerya purchasi) has been suppressed to non damaging levels on many important native host plants, including white mangrove, Acacia species Waltheria,Prosopis, Parkinsonia, Darwiniothamnus and Scalesia but less so on uva de mar and Rhynchosia minima. These plants are ecologically important species that were heavily attacked before the release of R. cardinalis. (3) In addition to being effective, the project has been safe, as no evidence from field observations or the large cage studies was found of attack by R. cardinalis on non-target insects.
A preliminary progress report on the biological control of Icerya purchasi by Rodolia cardinalis was submitted to the Charles Darwin Foundation in December 2009 and it can be downloaded. This report was translated into Spanish and submitted to the Galapagos National Park Service and SICGAL and the Spanish version is available for downloading. A powerpoint presentation capturing the major points of the biological control program was presented by Roy Van Driesche to the Galapagos National Park Service on December 11 2009 and this presentation in Spanish is available for downloading.
History of Biological Control of I.purchasi in California
In 1887, the fledgling citrus industry in California was threatened with destruction because of uncontrollable populations of I. purchasi. This pest was possibly introduced into California on Acacia plants imported from Australia in 1868 (this fact is uncertain, as records suggest that Acacia seeds and not plants were imported). Charles Valentine Riley, Chief of the Divsion of Entomology of the Federal Government addressed the Convention of Fruit Growers Meeting in Riverside California in April 1887 about the I. purchasi problem. During this presentation Riley stated that it would likely be a good idea to send a qualified entomologist to Australia "to introduce from Australia such parasites as serve to keep this fluted scale in check in its native land..." As a result of Riley's presentation the Convention adopted a resolution to send an entomologist to Australia to search for natural enemies of I. purchasi for importation into California.
To circumvent restrictions on the use of federal funds for travel (largely put in place to stop Riley's junkets to Europe), it was decided that a US Entomologist representing the State Department could attend an International Exposition in Melbourne as a Federal Government Representative. It was understood that the ulterior motive was to search for and collect natural enemies of I. purchasi. The Office of the United States Commissioner to the Exposition set aside $2,000 to pay for the expenses of the Entomologist selected to attend the Melbourne Exposition. Riley selected Albert Koebele, a naturalized German immigrant that Riley had appointed to work on insects in California in 1885.
|
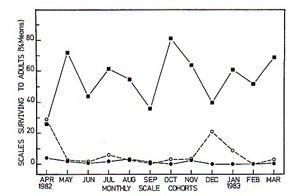
Graph 1. Effect of natural enemies on the survivorship of I. purchasi. When I. purchasi populations are protected from natural enemy attacks because of exclusion cages, populations of this insect become significantly higher in comparison to populations that are not protected from foraging natural enemies.
Graph produced by Y.K. Prasad (1989). |
Koebele discovered two natural enemies attacking I.purchasi in Australia, a parasitic fly, Cryptochaetum iceryae (Williston) and a predatory beetle, Rodolia cardinalis. Koebele sent 12,000 flies to California (this fly established and has provided control in the cooler coastal areas of California) and then commenced work on the beetle he had found feeding on I. purchasi in a north Adelaide garden on October 15, 1888. Koebele sent three consignments of beetles to D. W. Coquillett, an entomolgical field agent in Los Angeles; November 30, 1888 had 28 specimens, the second consigment arrived on December 29 with 44 beetles, and the third arrived January 24, 1889 with 57 specimens (total sent 129). The beetles were placed into a large tent that enclosed an I. purchasi citrus tree in the Wolfskill Orchard in Los Angeles. By April 12, 1889, the I. purchasi infestation on the enclosed citrus tree was reduced and the cage was opened to allow R. cardinalis to spread to adjacent trees. By June 12, 1889, 10,500 beetles had been collected from the Wolfskill Orchard and distributed to 228 citrus growers with I. purchasi infestations.
On February 21, 1889, Coquillett released 35 R. cardinalis onto trees owned by citrus grower J.R. Dobbins in the San Gabriel Valley. By July 2, 1889, Dobbins noted that R. cardinalis had multiplied so quickly on his 3,200 citrus trees that by June 1, 1889 he had distributed 65,000 beetles to 130 growers. On July 31, 1889, Dobbins posted a sign stating that he had no more R. cardinalis for removal because the I. purchasi populations had collapsed along with the abdundant R. cardinalis. Within ~18 months of the initiation of this project I. purchasi had been successfully controlled by R. cardinalis.
The shipments of fresh oranges from Los Angeles County jumped from 700 to 2,000 carloads within one year of the release of R. cardinalis and the total cost of the project was $1,500. In appreciation of Koebele's work, the California State Board of Horticulture presented Koebele with a gold watch and his wife with a pair of diamond earrings.
I. purchasi is still controlled in California with R. cardinalis. However, control has been upset periodically by the use of insecticides in citrus orchards. For example, use of DDT in the 1940's, organophosphates in the 1950's, carbamates in the 1970's, and over 1997-2002 neonicotinoids and insect growth regulators have disrupted biological control by R. cardinalis which allowed I. purchasipopulations to rebound to damaging levels.
Natural Control of I. purchasi in Australia
|
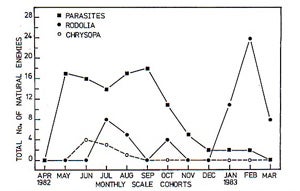
Graph 2. Changes in the phenology or population trends or abundance of key natural enemies that attackI. purchasi over time. When temperatures are cool in Adelaide, Australia (May to December) the parasitic fly C. iceryae is the dominant natural enemy. During the hotter parts of the year (January through March) R. cardinalis is the dominant natural enemy. Graph produced by Y.K. Prasad (1989). |
In Australia, I.purchasi is typically very rare and natural enemies are responsible for suppressing populations of I. purchasi in natural areas on native host plants (e.g., Acacia baileyana) (see Prasad 1989 in References section). The suppressive effect of natural enemies in Australia (and California) has been demonstrated through caging studies. When cages enclose cohorts of I. purchasi on tree branches and exclude natural enemies which eat or parasitize this insect, populations of I. purchasi increase. In the absence of natural enemies the average percentage larval to adult survivorship rates for I. purchasi are significantly greater in comparison to populations that are not protected from natural enemies. The two dominant natural enemy species attacking I. purchasi near Adelaide in South Australia are R. cardinalis and C. iceryae. The effect these two natural enemies have on I. purchasi varies throughout the year; C. iceryae dominates when temperatures are low (i.e., fall and winter) and over hotter periods (i.e., summer) R. cardinalis dominates. Quezada and DeBach (1973) (see References) also noted that in California C. iceryae is more effective in cooler coastal areas, while in warmer desert interiors R. cardinalis is more effective. In zones with intermediate temperatures, both natural enemies co-exist and their distributions over-lap in California.
Biology of Rodolia cardinalis
Adult R. cardinalis are hemispherical, 2.5-4.0 mm in length, and red and black in color. Females tend to be predominantly red in color, while males tend to exhibit more black. The intensity of the color patterns can be masked by fine body hairs which give adults a greyish appearance. Females are highly fecund and can lay 150-190 eggs each. Eggs tend to be laid either under adult I. purchasi, on top of adults within grooves, or rarely, female R. cardinalis will chew a hole in the ovisac and oviposit eggs inside the ovisac directly onto the developing eggs and crawlers of I. purchasi.
| Figure 9. An adult Rodolia cardinalis newly emerged from its pupal case. |
This predator has three larval stages and a pupal stage. The small larvae feed on I. purchasicrawlers, while larger larvae and adults will consume second and third instars and adult I. purchasi. Adults and large larvae can open the ovisac to feed on its contents. The development of R. cardinalis from egg to adult is fast, just 18 days at 25°C. This rapid development allows R. cardinalis to have 8-12 generations per year in comparison to the 3-4 generations I. purchasiwould undergo in areas with similar climatic conditions. Sustained temperatures approaching or exceeding 34°C are detrimental to egg hatching and larval survival rates, but adults can tolerate these conditions for periods of up to 72 hours.
Related Articles
Smithsonian Magazine: The Bug that Saved California
References |
- Ashmole, N.P. and Ashmole, M.J. 1997. The land fauna of Ascension Island: new data from caves and lava flows, and a reconstruction of the prehistoric ecosystem. Journal of Biogeography 24, 549-589
- Ben-Dov, Y., Miller, D.R. & Gibson, G.A.P. 2009. ScaleNet, Icerya purchasi. 12 August 2009. http://www.sel.barc.usda.gov/catalogs/margarod/Iceryapurchasi.htm
- Calderon Alvarez, C. 2002. Evaluación de la Eficacía de Rodolia cardinalis (Mulsant) (Coleoptera: Coccinellidae) en el Control Biológico de Icerya purchasi Maskell (Homptera: Margarodidae) en las Islas Galápagos. Tésis de grado. Pontifica Universidad Javeriana, Faculdad de Estudios Ambientales y Rurales, Bogota, Colombia.
- Caltagirone, L.E. and Doutt, R.L. 1989. The history of the vedalia beetle importation to California and its impact on the development of biological control. Ann. Rev. Entomol. 34, 1–16.
- Causton, C.E., 2001. Dossier on Rodolia cardinalis Mulsant (Coccinellidae: Cocinellinae), a Potential Biological Control Agent for the Cottony Cushion Scale, Icerya purchasi Maskell (Margarodidae). Charles Darwin Research Station, Galapagos Islands.
- Causton, C. E. 2003. Ensuring compatibility of biological control of Icerya purchasi Maskell with conservation in Galapagos: development of procedure to evaluate risk, pp. 448-457. In R. G. Van Driesche [ed.], Proceedings of the First International Symposium for the Biological Control of Arthropods. 14 -18 January 2002, Honolulu, HI. USDA-FS. FHTET-03-05, Morgantown, WV.
- Causton C.E., Lincango M.P., and Poulsom, T.G.A. 2004. Feeding range studies of Rodolia cardinalis (Mulsant), a candidate biological control agent of Icerya purchasi Maskell in the Galápagos Islands. Biological Control 29 (3): 315-325.
- Causton, C.E., S.B. Peck, B.J. Sinclair, L. Roque-Albelo, C.J. Hodgson, B. Landry. 2006. Alien insects: threats and implications for the conservation of the Galápagos Islands. Annals of the Entomological Society America 99: 121-143.
- Cronk, Q.C.B., 1980. Extinction and survival in the endemic vascular flora of Ascension Island. Biological Conservation 17: 207–219.
- Doutt, R.L. 1964.Historical Development of Biological Control. Pages 21-42. In: Biological Control of Insect Pests and Weeds, Eds: P. DeBach and E. I. Schlinger. Reinhold Publishing Corporation, New York
- Ebeling W. 1959. Subtropical Fruit Pests. University of California Press, Los Angeles.
- Fowler, S.V. 2004. Biological control of an exotic scale, Orthezia insignis Browne (Homoptera: Ortheziidae), saves the endemic gumwood tree, Commidendrum robustum (Roxb.) DC. (Asteraceae) on the island of St. Helena. Biological Control 29: 367-374.
- Grafton-Cardwell, E.E., Gu, P., and Montez, G.H. 2005. Effects of temperature on development of vedalia beetle, Rodolia cardinalis (Mulsant). Biological Control 32: 473-478.
- Hale, L. D. 1970. Biology of Icerya purchasi and its natural enemies in Hawaii. Proc. Haw. Ent. Soc. 20: 533-550.
- Hill, M.G. and Newbery, D.M. 1980. The distribution and abundance of the coccid lcerya seychellarum Westw. on Aldabra atoll. Ecological Entomology 5: 115-122.
- Johnson, S. and R. Threadgold. 1999. Report on the monitoring and status of the coccid (Icerya seychellarum) on Aldabra from 1980 to 1999. Unpublished report, Seychelles Islands Foundation.
- Mendel, Z., Blumberg, D., Zehavi, A., and Weissenberg, M. 1992. Some polyphagous Homoptera gain protection from their natural enemies by feeding on toxi plants Spartium junceum and Erythrina corallodendrum (Leguminosae). Chemoecology 3: 118-124.
- Newbery, D.M. and Hill, M.G.1985. Changes in the distribution of the coccid Icerya seychellarum Westw. on Aldabra atoll in relation to vegetation density. Atoll Research Bulletin No. 291.
- Newbery, D.M. 1988. Recently monitored trends in the abundance of Icerya seychellarum Westw. (Insecta: Homoptera) on Aldabra Atoll, with suggestions for its biological control. Bull. Biol. Soc. Wash. 8: 30-39.
- Niznik, S., and Szklarzewicz T. 2006. Organizaton of the hermaphroditic gonad and transovarial transmission of endosymbiotic bacteria in Icerya purchasi (Insecta, Hemiptera, Coccinea: Monophlebidae). ACTA Biologica Cracoviensia Series Botanica. 48 (supplement 1): 23.
- Prasad, Y.K. 1989. The role of natural enemies in controlling Icerya purchasi in South Australia. Entomophaga 34: 391-395.
- Quezada, J.R., and DeBach P. 1973. Bioecological and population studies of the cottony-cushion scale, Icerya purchasiMask., and its natural enemies, Rodolia cardinalis Mul. and Cryptochaetum iceryae Will., in Southern California. Hilgardia 41: 631-638.
- Roque-Albelo, L. 2003. Population decline of Galapagos endemic Lepidoptera on Volcan Alcedo (Isabela island, Galapagos Islands, Ecuador): an effect of the introduction of the cottony cushion scale? Bull. Inst. R. Sci. Nat. Belg. Entomol. 73: 1-4.
- Tort, N. 2004. A study on some anatomical parameters of the piercing-sucking process in leaves and branches of Pittosporum tobira L. (Pittosporaceae) infested by the cottony cushion scale, Icerya purchasi Maskell (Homoptera: Coccina, Margarodidae). Journal of Pest Science 77: 53-56.