Authors: Mari West, Marco Molfini, Francesc Gomez Marco, Mark Hoddle
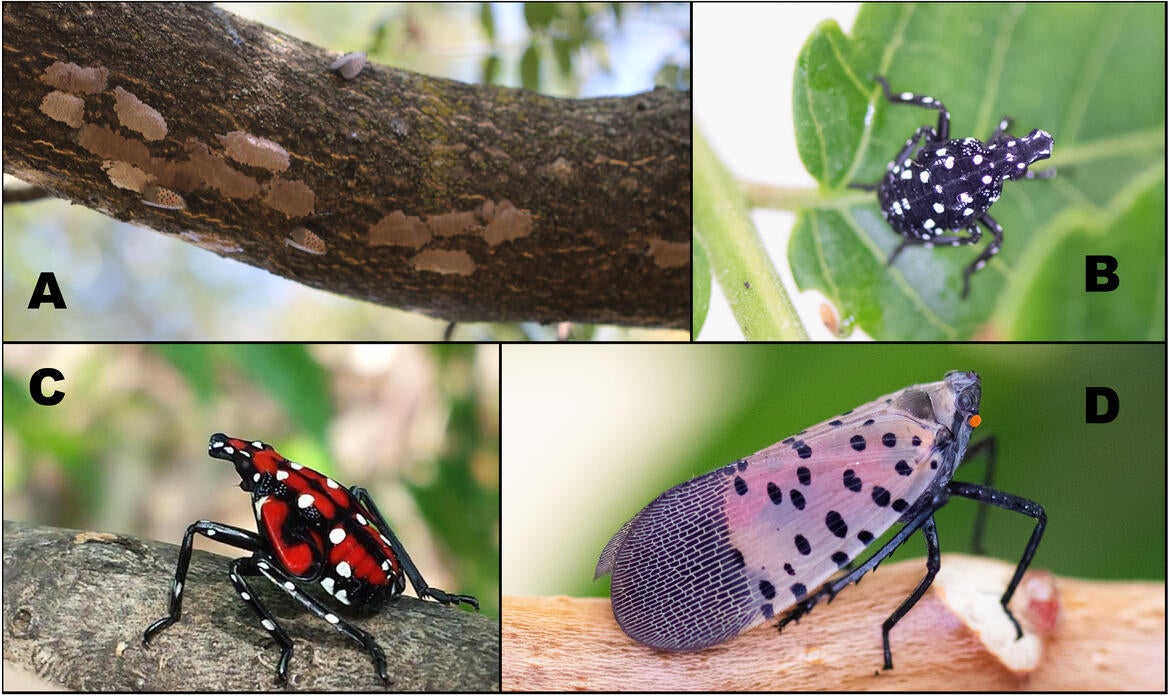
Spotted Lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae)
The Problem
The spotted lanternfly, Lycorma delicatula, is an invasive sapsucking insect, originally from parts of Asia, that was first detected in Pennsylvania in 2014. It has rapidly developed high density populations and spread to new areas in the US, with active infestations present as far west as Illinois and as far south as Georgia (as of Jan. 2025; NYSIPM). The spotted lanternfly can feed on over 100 species of woody vines and trees. Within invaded regions, spotted lanternflies are significant pests of many crops, ornamentals, and native forests. One of its preferred host plants, tree of heaven (Ailanthus altissima), a widespread invasive weed, provides a reliable food source across much of the US and may help spotted lanternflies to establish in newly invaded areas.
While spotted lanternfly nymphs and adults can disperse to nearby areas through walking, hopping, and flying, unintentional transportation of spotted lanternfly egg masses related to human activities is probably most responsible for long-distance introduction into new areas. Spotted lanternfly egg masses, laid on tree-bark and observed from September to June, can also be laid on many different natural and man-made materials, including outdoor furniture, building materials, vehicles, firewood, etc. Eggs attached to these inert materials can easily be moved over long distances into new areas and “hitchhiking” in this manner increases the difficulty of detecting and destroying spotted lanternfly egg masses. It is suspected that the initial introduction of spotted lanternfly into Pennsylvania occurred when a shipment of paving stones harboring egg masses was imported from China. Since then, isolated spotted lanternfly populations have appeared throughout the eastern US, likely through similar accidental transportation of eggs.
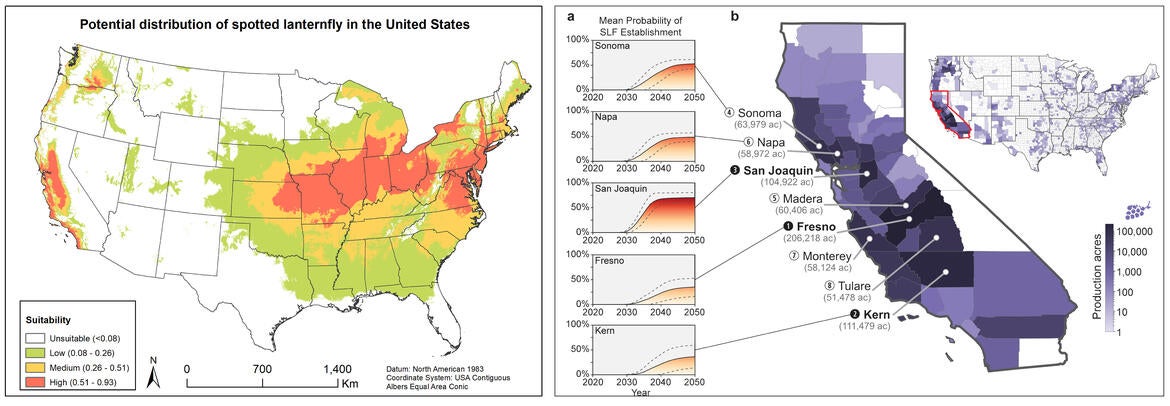
If given the opportunity, ecological niche (i.e., species distribution) modeling predicts that spotted lanternfly is likely to establish in California (Wakie et al. 2019, Jones et al. 2022). Although there is currently no known active infestation in California, border control stations have intercepted spotted lanternfly egg masses on incoming firewood (CDFA) and outdoor sculptures (CDFA PD-GWSS) on several occasions, and invasion of California within the next decade is highly anticipated. The pest poses a significant threat to CA agricultural industries, with the potential to cause significant damage to the grape, citrus, pome, and stone fruit industries, which are collectively worth over $9 billion USD (CDFA 2023).
Currently, control of spotted lanternflies in infested areas of the US relies heavily on the use of insecticides. The majority of these insecticides work well against spotted lanternflies and applications can reduce infestation severity. However, because the pest can feed on many plant species, it often occupies habitat in surrounding urban, suburban, and forested areas, from which it readily reinfests insecticide treated areas, once residues are no longer lethal. This has led to repeated application cycles of insecticides, which can increase management costs, pose risks to environmental and human health, and potentially lead to spotted lanternflies developing insecticide resistance. For this reason, several ongoing studies are exploring alternative solutions to the use of insecticides to control spotted lanternfly.
Our Research
Proactive Biological Control of Spotted Lanternfly
Much of our spotted lanternfly work has focused on applied biological control – a management strategy that uses ‘natural enemies’ (e.g., predators, parasites, parasitoids, and pathogens) to suppress high density pest populations to less damaging levels. Implementation of effective biological control programs reduces reliance on insecticides for pest control, which lessens economic and environmental costs and impacts associated with the management of damaging, invasive pests. Our research, together with that of our collaborators at USDA-APHIS, explores the possibility of using parasitoids to control the spotted lanternfly, with the goal of implementing a successful biological control program. Parasitoids are small wasps that lay their eggs inside the eggs or in or on the bodies of their hosts (in this case, spotted lanternfly). Developing parasitoids consume and eventually kill their hosts as they grow. When developing parasitoids reach maturity, they emerge from their hosts, mate, locate additional hosts to parasitize, and this lifecycle is repeated. If natural enemy population growth and negative effects on the target pest population are sufficient enough, target pest population densities decline and become less damaging. In this way, successful biological control programs are often self-sustaining, after initial release of parasitoids in pest-infested areas, and reliance and use of insecticides for pest control can be greatly reduced.
Classical biological control programs (see below for more details on classical biological control programs) often involve the importation, mass-rearing, and release of co-evolved natural enemies from a pest’s native range. This process requires approval from Federal and State governments and coordination with local agencies. In order to get a natural enemy approved for release into a new area, safety testing must first be undertaken to ensure that the release of biocontrol agents reared in a secure quarantine facility will not harm local species or drastically disrupt the balance of the local ecosystem. Safety tests include determining which species of non-target hosts the proposed natural enemy will kill and/or reproduce on (i.e., host range testing) and whether the natural enemy of interest exhibits a strong preference for the target pest in comparison to non-target host species (i.e., assessment of host specificity).
Additional information that can be used to determine the potential efficacy of a new natural enemy species for use in a biological control program targeting an invasive insect pest could include assessing how effectively the natural enemy controls the target pest in its native range (or other areas where it may have already been introduced to control the same pest species), estimating the potential ability of the natural enemy to establish viable populations in infested areas, and evaluating the potential for spread into new areas from these points of establishment.
Because of the rigorous testing (i.e., host range and host specificity testing) and regulatory agency (i.e., USDA-APHIS) approvals required before natural enemies can be released from secure quarantine facilities into areas with target pest populations, biological control programs often require years of work (typically > than 3 years for insect natural enemies, and up to 10 years or more for natural enemies that attack weeds) to implement.
Thus, when we can anticipate the arrival of a damaging pest, like the invasion of spotted lanternfly into California, it is critical to take a proactive approach to prepare for this likely incursion and mitigate the likely negative effects of the invasion as much as possible.
Consequently, the goal of our proactive biological control research targeting spotted lanternfly prior to its anticipated invasion into California has been to identify an effective and safe natural enemy of the pest, complete all necessary safety testing to ensure environmental risks are minimized, and to have all the necessary approvals in place for release and establishment of the candidate natural enemy before spotted lanternfly establishes in California.
This proactive approach ensures that the biological control program is ready to be enacted as soon as possible, most likely once it has been determined that populations of spotted lanternfly cannot be contained or eradicated. A rapid response focused on the target pest with pre-approved biological control agents may significantly reduce rates of pest population growth in infested areas, and as a result of this population suppression, rates of spread may also be reduced because fewer pests are available to move into new areas where they could establish.
Proactive spotted lanternfly research in California was conducted in UC Riverside’s Insectary and Quarantine Facility, which is designed to contain small biological organisms, like pest insects and their natural enemies. This highly secure containment facility allows us to conduct controlled laboratory experiments, while ensuring that neither the spotted lanternflies nor any of the natural enemies that we are testing are able to escape.
Classical Biological Control of Spotted Lanternfly
The deliberate importation and release of natural enemies from a pest’s native range to control the pest in its invasive range is known as ‘classical’ or ‘introduction’ biological control. Two parasitoid species are known to attack spotted lanternfly in parts of its native range in China – Anastatus orientalis, an egg parasitoid (attacks eggs), and Dryinus sinicus, a nymphal parasitoid (attacks nymphs). Both are being assessed for potential use in classical spotted lanternfly biological control programs in the US.
Anastatus orientalis
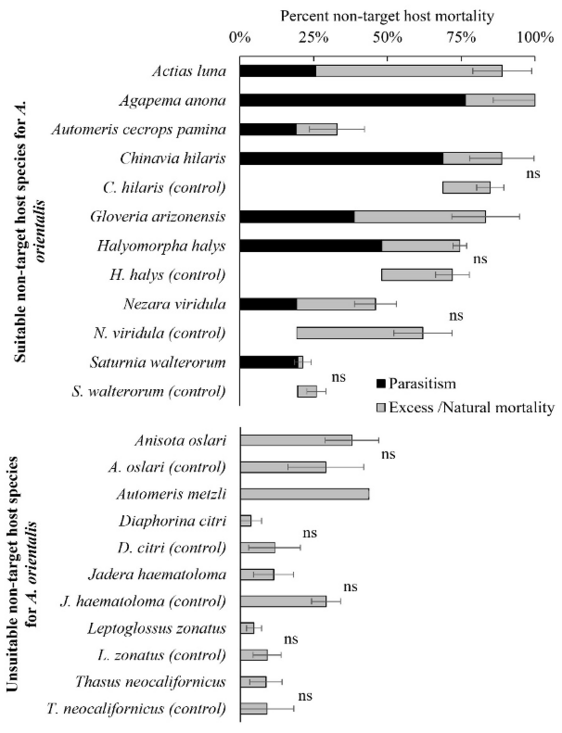
Anastatus orientalis is an egg parasitoid that is known to parasitize spotted lanternfly eggs in northern China and it has been utilized in the Republic of Korea (i.e., South Korea) in a classical biological control program targeting invasive populations of spotted lanternfly. In order to determine if A. orientalis is a suitable candidate for a spotted lanternfly biological control program in California, we assessed its ability to attack and kill eggs of various non-target host species, including moth and true bug species native to the western US. The goal of this work was to determine if releasing A. orientalis in California would pose a significant threat to any of the native species that A. orientalis could encounter in nature.
Unfortunately, this parasitoid successfully parasitized eggs of many different species, including those of native hemipteran (i.e., native stink bugs) and lepidopteran (i.e., moths) hosts. Work carried out by researchers at USDA-APHIS using host species native to the eastern US had similar results. Unfortunately, because A. orientalis has a wide host range and poses a threat to native non-target species, this egg parasitoid from China is unlikely to be approved for release in the US as part of a classical biological control program for spotted lanternfly. For more detailed information about our work on A. orientalis, check out Gómez Marco et al. 2023 and Gómez Marco and Hoddle 2024.
Dryinus sinicus
Dryinus sinicus is a nymphal parasitoid known to attack, feed on, and parasitize spotted lanternfly nymphs in China. Collaborators at USDA-APHIS are working with this parasitoid to determine if it is a suitable candidate for use in a classical biological control program for spotted lanternfly in the US. An important aspect of this assessment is host range testing of D. sinicus, with a particular focus on US native non-target host species. California (i.e., the Applied the Biological Control Lab at UC Riverside) is contributing to this work being carried out by USDA-APHIS by collecting egg masses and nymphs of western US native lanternflies (Fulgoridae) and other related planthoppers to include in non-target host-range testing.
Biological Control of Spotted Lanternfly with Native Natural Enemies Found in the Western US
An alternative to importing parasitoid species that attack spotted lanternfly in its native range is to assess the ability of Western US native parasitoids to control spotted lanternfly. Native parasitoids may not only be able to offer some level of biotic resistance to spotted lanternfly invasion, lessening invasion severity, but might also be used for targeted release in newly invaded areas. Using native parasitoids as part of biological control program, while still requiring careful consideration, may be less risky to native non-target host species, especially if primarily utilized in developed urban areas where spotted lanternfly is likely to first establish. Using native parasitoids for spotted lanternfly biological control may also benefit from a faster, simpler approval process by regulatory agencies as these natural enemies are already present in the environment, and a biological control program would simply boost existing numbers of these agents and concentrate releases in areas infested with spotted lanternfly. There are several native species of Anastatus egg parasitoids in California, and the US in general, that may be suitable for use in spotted lanternfly biocontrol programs. Our work has identified some promising native candidate natural enemies.
Anastatus reduvii
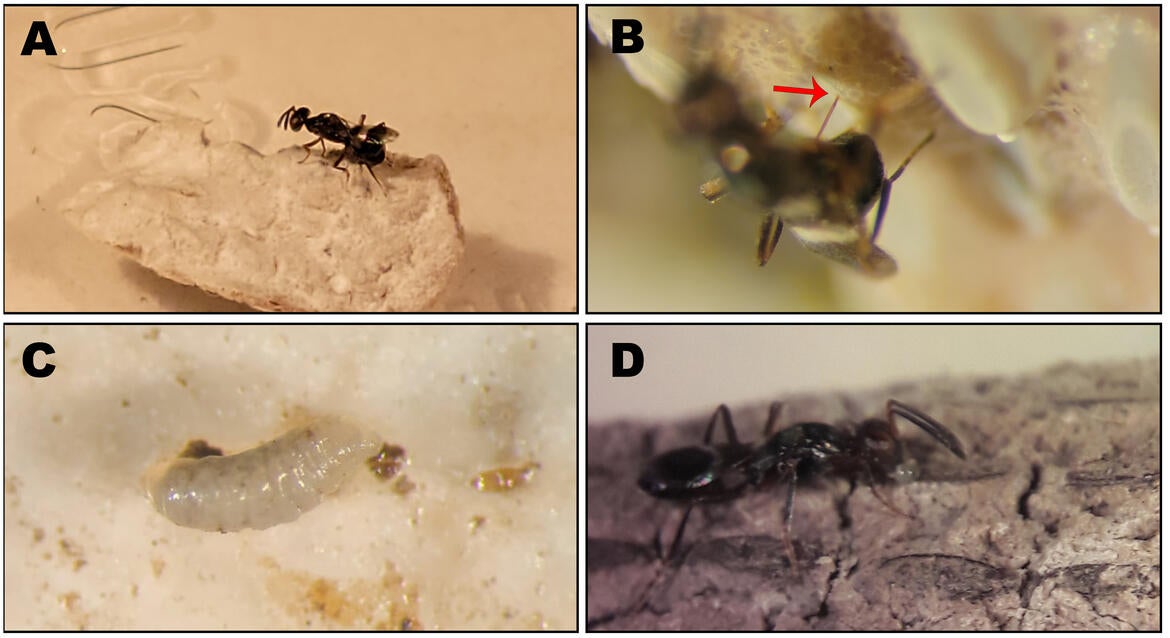
We evaluated Anastatus reduvii, a widely distributed North American egg parasitoid, for its potential as a biological control agent for the spotted lanternfly. Native to numerous regions, including California, A. reduvii reproduces parthenogenetically, with most offspring being female, and males produced rarely. This parasitoid has been found emerging from field-collected spotted lanternfly egg masses in the eastern US, suggesting it could be an effective natural enemy for use in a biological control program targeting spotted lanternfly.
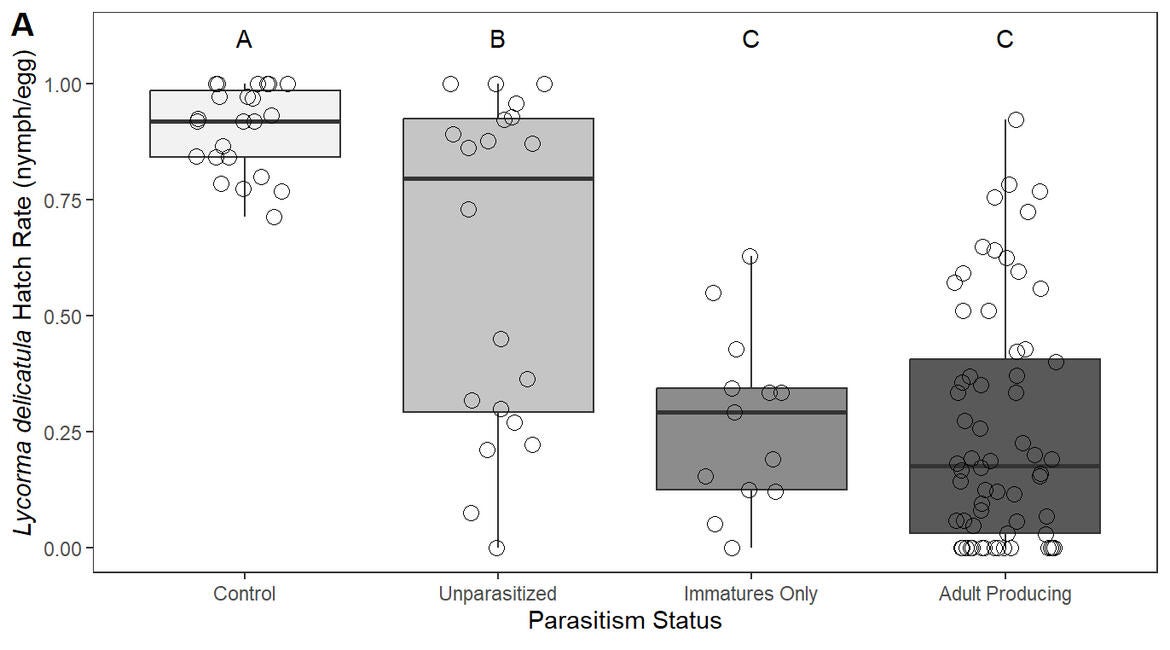
In the UCR quarantine facility, lab experiments showed that spotted lanternfly egg masses exposed to A. reduvii had a significantly lower survival rate (just 33%) compared to the 90% survival rate of unexposed egg masses (West et al. 2024). The mortality of spotted lanternfly eggs was due to a combination of factors, including successful parasitism and possible host feeding.
Host feeding occurs when female parasitoids consume host eggs (in an act of predation) to obtain the necessary protein for egg development within their ovaries. While parasitism results in the death of the host egg and the emergence of new adult parasitoids, host feeding also kills host eggs, but does not lead to the production of new parasitoids. Therefore, parasitism results in the reproduction of parasitoids and the death of the host, while host feeding, results in host death without parasitoid reproduction.
The combined effect of parasitism and host feeding by female A. reduvii could lead to significant reductions in spotted lanternfly population densities. By engaging in both behaviors, female parasitoids contribute not only to the control of pest populations but also to the growth of their own populations, which could enhance natural pest management over time as parasitoid populations grow and spread, possibly tracking spotted lanternfly (i.e., accidental movement of parasitized spotted lanternfly eggs) as it moves into new areas.
As of January 2025, the California Department of Food and Agriculture (CDFA) and USDA-APHIS, were maintaining colonies of A. reduvii for possible future use in biological control programs targeting spotted lanternfly.
The search for additional native species of parasitoids that attack the eggs of native lanternfly species
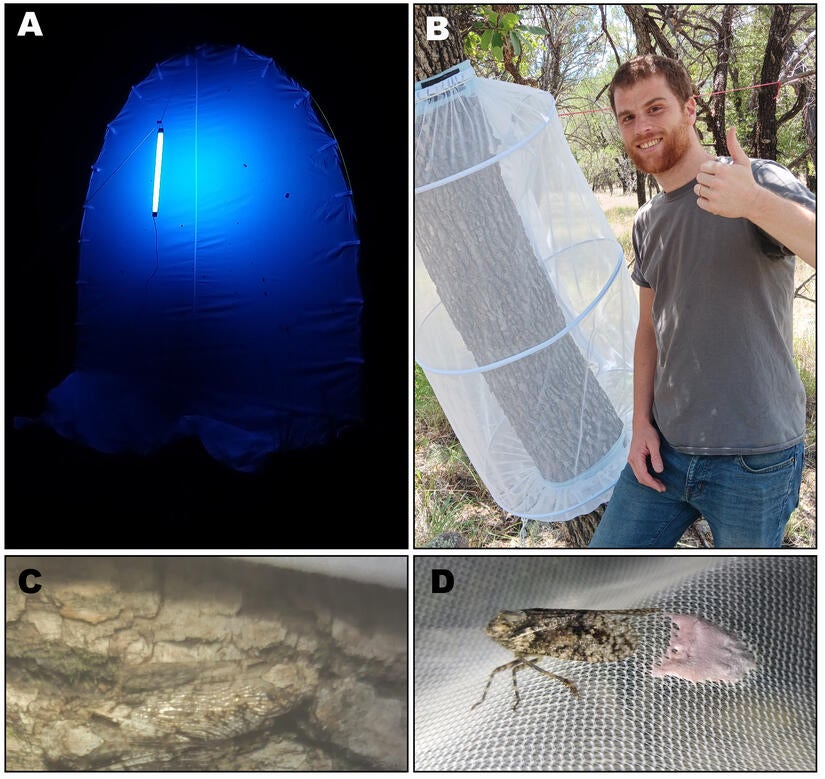
The search for additional species of native US parasitoids with the potential to attack eggs of spotted lanternfly was confronted with an immediate and crucial challenge: identifying parasitoids of native US lanternflies! At the start of our project, no data were available on native parasitoids attacking the eggs of native lanternflies. Although many fulgorid species have been collected across the US, particularly in the southwestern regions like Arizona's Sky Island mountains, many of these species were undescribed and little was known about their biology (e.g., What host plants do they feed on? When and where do they lay eggs?), which posed considerable challenges for our proactive research program on spotted lanternfly.
To address these knowledge gaps, we launched a preliminary investigation to identify native fulgorids. To do this work, we performed blacklight sampling at the Southwestern Research Station (SWRS) in the Chiricahua Mountains, AZ during the monsoon seasons from 2018 to 2024. During this fieldwork, we identified and described new species of Fulgoridae (Yanega et al. 2024). In August 2023, adults of two newly described fulgorid species (Scaralina aethrinsula and S. cristata) were collected and reared in mesh cages placed on their host plant, Quercus arizonica (Arizona oak).
From these caged rearings on native oak, we collected 35 native lanternfly egg masses, which were then attached to Arizona oaks as "sentinel egg masses." These artificially deployed egg masses essentially acted as “baited traps” to attract local, native egg parasitoids. After exposure periods ranging from 15 to 40 days, we collected the egg masses and stored them in an incubator at the UC Riverside Insectary and Quarantine Facility. In total, 46 parasitoids emerged from four sentinel egg masses. These included two new, undescribed species of Anastatus, and one of these previously unknown native parasitoid species successfully parasitized spotted lanternfly eggs. This positive finding is suggestive that this newly discovered native egg parasitoid may be a good candidate for further investigation as a biological control agent for spotted lanternfly.
Additional Resources:
- Gomez-Marco, F., D. Yanega, M. Ruiz, and M.S. Hoddle. 2023. Proactive classical biological control of Lycorma delicatula (Hemiptera: Fulgoridae) in California (U.S.): Host range testing of Anastatus orientalis (Hymenoptera: Eupelmidae). Frontiers in Insect Science DOI: 10.3389/finsc.2023.1134889
Gómez-Marco, F. and M.S. Hoddle. 2024. Proactive biological control of spotted lanternfly: parasitism and host feeding behavior of Anastatus orientalis (Hymenoptera: Eupelmidae) on Lycorma delicatula (Hemiptera: Fulgoridae) egg masses. Biological Control 195: 105551.
- Hoddle, M.S. 2019. Spotted Lantern Fly is Coming – California is Getting Ready, Now! CAPCA Adviser 22(4): 38-41.
- Molfini, M.*, M. West*, F. Gómez-Marco, J.B. Torres, and M.S. Hoddle. 2024. Is Lycorma delicatula (Hemiptera: Fulgoridae) a blooming threat to citrus? Journal of Economic Entomology https://doi.org/10.1093/jee/toae197
- Molfini, M.*, M. West*, F. Gómez-Marco, A. Iacovone, and M.S. Hoddle. 2025. Proactive evaluation of a native European parasitoid, Anastatus bifasciatus (Hymenoptera: Eupelmidae), for biological control of Lycorma delicatula (Hemiptera: Fulgoridae). Biological Control, p.105730. https://doi.org/10.1016/j.biocontrol.2025.105730
- West, M.*, M. Molfini*, M.S. Hoddle. 2025. Proactive assessment of a native North American egg parasitoid, Anastatus reduvii (Hymenoptera: Eupelmidae), as a biological control agent of Lycorma delicatula (Hemiptera: Fulgoridae), in California. Biological Control 105687 https://doi.org/10.1016/j.biocontrol.2024.105687
- Yanega, D., G. Goemans, M.V. Dam, F. Gómez-Marco, M.S. Hoddle. 2024 Description of a new genus of North and Central American planthoppers (Hemiptera: Fulgoridae) with fourteen new species. Zootaxa. 5443(1):1-53. https://doi.org/10.11646/zootaxa.5443.1.1
*authors contributed equally