Biological Control of the Glassy-winged Sharpshooter (Homalodisca vitripennis) in California
Prepared by the Applied Biological Control Research Laboratory. Contact: Nic Irvin, Biological Control Specialist and Research Scholar
nic.irvin@ucr.edu
Introduction |
|
Figure 1. Adult glassy-winged sharpshooter. |
After a significant lag phase, extraordinary population growth occurred about 10 years after its successful establishment in California. This was facilitated, in part, by a lack of co-evolved natural enemies in the invaded area, coupled with irrigation of agricultural and urban areas in desert habitats normally too dry to support GWSS populations (Hoddle 2004a), and possibly adaptation to the environment of Southern California.The glassy-winged sharpshooter (GWSS), Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae), is native to the southeastern United States and northeastern Mexico. It is thought that GWSS invaded and established in southern California sometime around 1990 (Sorensen and Gill 1996). It has since become widely established in several counties across southern California (CDFA 2003). GWSS has also successfully invaded French Polynesia (the Society Islands, Marquesas and Austral Island groups), [established 1999 (Cheou 2002)], Hawaii [established 2004 (Hoover 2004)], Easter Island [established 2005 (Sandra Ide pers. comm.)], and the Cook Islands (established 2007 [Disna Gunawardana pers. comm.]).
| Figure 2. Almond leaf scorch. |
| Figure 3. Oleander leaf scorch. |
GWSS has piercing-sucking mouthparts, and nymphs and adults feed exclusively on xylem fluids. Adults consume 1.8 - 2.9 ml of fluid per day on cowpea and 0.2ml - 4.5 ml when feeding on citrus. The ability of GWSS to spread the xylem-dwelling plant pathogenic bacterium Xylella fastidiosa is the key reason GWSS is classified as a serious pest in California and elsewhere. Across California, GWSS has vectored X. fastidiosa, the causative agent of Pierce’s disease (PD), on grapes, almond leaf scorch, alfalfa dwarf, and oleander leaf scorch. The number and type of plant diseases caused by X. fastidiosa vectored by GWSS is likely to increase as new hosts expressing X. fastidiosa induced diseases are identified. The bacterium has already been found to be the causative agent of two previously unrecognized diseases in olive trees and liquidambar. Table 1 lists a number of X. fastidiosa strains and their ornamental host sources (extracted from Hernandez-Martinez et al. [2007]). In South America, strains of X. fastidiosa cause disease in citrus (citrus variegated chlorosis) (Hartung et al. 1994) and coffee (coffee leaf scorch) (de Lima et al. 1998). Recently in Costa Rica, X. fastidiosa has been associated with disease in avocados (Montero-Astúa et al. 2008).
The GWSS-X. fastidiosa combination has devastated or has the potential to adversely affect many agricultural crops, urban ornamental and landscape plants, and possibly native vegetation. The potential effect of GWSS vectoring X. fastidiosa into native vegetation in California that previously has had no prior association with the bacterium is particularly worrisome as it may lead to new disease epidemics not previously seen. Consequently, the establishment of GWSS in California has irrevocably changed the ecology of X. fastidiosa in California’s wilderness, agricultural, and urban landscapes.
There are currently no records of X. fastidiosa in French Polynesia, Hawaii, Easter Island and the Cook Islands. It is possible the bacterium has been introduced into these South Pacific Islands by means of the importation of ornamental plants from areas in the Americas where X. fastidiosa is native. These potential silent X. fastidiosa reservoirs may harbor bacteria without expressing disease symptoms and, with the arrival of a vector such as GWSS, pathogen transmission to susceptible host plants could conceivably occur.
Economic Impact |
The economic cost to California caused by GWSS-X. fastidiosa is immense. Oleander leaf scorch has been estimated to have caused damage in excess of $52 million along 2,000 miles of freeway median plantings (Costa et al. 2000). In 2000, $6.9 million was spent on pesticides for area-wide spraying of GWSS habitats in an effort to manage populations migrating into vineyards in Temecula and Bakersfield, California. Grape growers in Riverside and San Diego counties in 1998 and 1999 accrued estimated losses of $37.9 million because of GWSS-X. fastidiosa related diseases (Siebert 2001).
| Figure 4. Damage caused by Pierce's Disease (PD) to grapes, transmitted by GWSS. |
In 2002, primary producers incurred additional economic costs resulting from containment activities such as inspections of export nursery stock and shipments of bulk grapes and citrus from H. vitripennis infested counties (CDFA 2003).
In 2008, it was reported that GWSS-transmitted Pierce’s Disease threatened $4 billion of grape vine production, and another $52 billion of California’s economy associated with the grape industry. Damage caused by GWSS-X. fastidiosa also threatens other crops, such as almonds ($2.8 billion), citrus ($1.1 billion), and stone fruit ($1 billion); ornamental shade trees are also at risk. A study conducted by the University of California found that between 1994 and 2000, Pierce’s disease caused nearly $30 million in losses and destroyed over 1,000 acres of grape vines in Northern California (CDFA 2008).
In 2003, there were in excess of 70 research programs on H. vitripennis or X. fastidiosa in California. Because of the serious nature of this problem and the vast sums of money at stake, the National Academy of Sciences (NAS), a United States society of distinguished scholars engaged in scientific and engineering research with a mandate requiring it to advise the Federal Government on scientific and technical matters, has subjected these research programs to evaluation and assessment (CDFA 2003). In 2009/2010, the number of GWSS-X. fastidiosa related research programs was 64 (Esser and West 2010).
Classical Biological Control |
Researchers at the University of California at Riverside (UCR), United States Department of Agriculture’s Agricultural Research Service (USDA-ARS) and the California Department of Food and Agriculture (CDFA) have pursued classical biological control strategies to reduce populations of GWSS in California. Natural enemies, in particular egg parasitoids, deemed safe and cleared from secure quarantine facilities were released into the environment where they attacked GWSS eggs. Female parasitoids lay their eggs inside GWSS eggs and the developing parasitoid larvae kill GWSS eggs by feeding inside the GWSS egg. The parasitoid larvae pupate inside the GWSS egg and then chew a circular exit hole through which they emerge. The winged parasitoids can fly, and after mating they search out more GWSS eggs to parasitize. In this manner, GWSS egg parasitoids help regulate pest population growth and subsequent abundance without the need for insecticides.
Foreign Exploration for Natural Enemies in the Pests' Home Range
| Figure 5. Gonatocerus ashmeadi |
| Figure 6. Gonatocerus triguttatus |
In the southeastern USA and northeastern Mexico, GWSS eggs are parasitized by several species of mymarid and Trichogrammatid parasitoids. Gonatocerus ashmeadi Girault, G. triguttatus Girault, G. morrilli Howard, and G. fasciatus Girault, all species of parasitoid in the family Mymaridae (Order Hymenoptera), are the most common natural enemies associated with H. vitripennis eggs in the southeastern United States (Triapitsyn and Phillips 2000). In an effort to use natural enemies to control invasive GWSS populations in southern California, these parasitoids were imported from the southeastern states, cleared through quarantine, and introduced into urban and agricultural areas. While G. morrilli is native to California, G. ashmeadi is self-introduced into California from the southeast USA and may have established on incipient GWSS populations or, more likely, on the native smoke-tree sharpshooter, Homalodisca liturata Ball, (Vickerman et al. 2004). Parasitoids from the native range of GWSS in the southeastern US have been introduced to bolster populations of resident species because their establishment may increase the efficacy of resident populations of control agents. For example, it is possible that different populations, while the same species with a different genetic constitution, may exhibit greater ability to tolerate high temperatures, dry conditions, or have different host plant preferences for foraging. The genetic variability of resident G. ashmeadi and G. morrilli populations in California have been increased through the release of new stock by the CDFA and this, in turn, may lead to improved biological control. In addition, new species of GWSS egg parasitoids have been introduced into California, and these include Gonatocerus triguttatus and Gonatocerus fasciatus. G. triguttatus was imported from eastern Texas and released in California in 2001. G. fasciatus was imported into California from Louisiana and released in 2002 (CDFA, 2003). Establishment and recovery of G. triguttatus and G. fasciatus has been inconsistent. For example, of the 69,474 G. triguttatus that were released in 2008, only ~33 parasitized H. vitripennis egg masses were recovered for this species in 11 out of 65 release sites (17%). Similar poor results have resulted for G. fasciatus and mass production and release of this parasitoid was discontinued due to poor recovery rates (CDFA, 2008).
Competitive Ability and Biological Control Potential of Gonatocerus Parasitoids
| Figure 7. Gonatocerus morilli |
| Figure 8. Gonatocerus fasciatus on a parasitized GWSS egg mass |
Interspecific competition between G. ashmeadi, G. triguttatus and G. fasciatus for GWSS egg masses was investigated in the laboratory by Irvin and Hoddle (2005) using three different experimental designs. Results showed that overall parasitism by G. ashmeadi was consistently higher (up to 76.0%) compared with G. triguttatus and G. fasciatus for all three studies. This suggests that female G. ashmeadi show greater potential as a biological control agent of GWSS and could out-compete G. triguttatus and G. fasciatus in the field and hinder their successful establishment and impact in California. Establishment and recovery of G. triguttatus and G. fasciatus in the field has been very low (see above) and the results of these experiments may suggest why this is the case; G. ashmeadi is too strong a competitor and has successfully excluded these other species.
Parasitism by G. fasciatus was consistently significantly lower (17.4-76.0% lower) than both G. ashmeadi and G. triguttatus for all three experimental studies. Gonatocerus fasciatus may have performed poorly because:
- This species is gregarious in nature and smaller in size compared with G. ashmeadi and G. triguttatus, therefore female G. fasciatus may require a longer period of time for each individual host handling.
- Smaller eggs oviposited by female G. fasciatus conceivably give rise to smaller larvae which may be less competitive when host eggs are attacked by more than one parasitoid species. The species with the bigger larvae will win fights inside the eggs.
- Gonatocerus fasciatus larvae probably do not participate in larval combat, since gregarious larvae often frequently contact one another under normal development (Salt, 1961), therefore they may be at a competitive disadvantage when another species of parasitoid also attacks the same host egg.
Alternatively, the competitive inferiority of G. fasciatus may simply be due to subordinate behavior when competing for egg masses with congeneric species. Results from a study conducted by Irvin and Hoddle (2005) which recorded visual observations on the behavior of one female G. ashmeadi, G. triguttatus and G. fasciatus simultaneously foraging on one GWSS egg mass showed that G. fasciatus allocated a significantly higher proportion (31.5%) of time to resting/grooming, compared with oviposition, whereas female G. ashmeadi allocated equal time to both activities. This may suggest that female G. fasciatus are less aggressive than G. ashmeadi and G. triguttatus when foraging for host eggs and they avoid conflict with larger parasitoids. Furthermore, 39.6% of time allocated by female G. fasciatus was spent off leaves with GWSS egg masses, and it was observed that G. ashmeadi and G. triguttatus often aggressively protected the GWSS egg mass, sometimes excluding access by G. fasciatus.
New Association Biological Control |
A co-evolved natural enemy may be more efficient in finding and attacking a target pest because it has evolved to exploit it (Messenger and van der Bosch 1971). Alternatively, it has been argued that biological control agents that have not co-evolved with a pest will be more effective natural enemies because coevolution between pests and biological control agents leads to decreased effectiveness of natural enemies and increased resistance of the pest to attacks because the system works towards establishing a balance between these two entities (Pimentel 1963). Consequently, it has been proposed that new association biological control agents which have no evolutionary history with the target pest should be used because the pest will be highly vulnerable to attack by this novel agent. This form of biological control with non-co-evolved natural enemies is called ‘new association’ biological control (Hokkanen and Pimentel 1989).
Gonatocerus tuberculifemur
Gonatocerus tuberculifemur is a common and widespread parasitoid that attacks eggs of Proconiini sharpshooters in Argentina and Chile in South America. It was imported from Argentina into quarantine in Texas in 2001, and into California in 2002, and reared on egg masses of H. vitripennis, (Triapitsyn et al., 2008). G. tuberculifemur has no evolutionary history with H. vitripennis and if released into California, this would make G. tuberculifemur a “new association” biological control agent of H. vitripennis.
Quarantine studies were performed to determine G. tuberculifemur’s viability and potential to be introduced into California as a biological control agent. Trials were conducted to compare egg age preference, competitive ability, and behavior between G. tuberculifemur and G. ashmeadi, the dominant parasitoid of California. They showed that G. ashmeadi consistently outperformed G. tuberculifemur in varying experimental arenas, and parasitized between 25% and 50% more eggs than G. tuberculifemur across varying host densities, egg ages, and exposure times (Irvin et al. 2009, Irvin and Hoddle 2010). G. ashmeadi consistently outperformed G. tuberculifemur by demonstrating an ability to exploit a larger host egg age range and produced more female offspring when compared with G. tuberculifemur (Irvin and Hoddle 2010). The ability to parasitize more host eggs over a wider range of host egg age categories is a favorable trait because it increases the probability that H. vitripennis eggs of varying ages will be successfully parasitized and may enable G. ashmeadi to outcompete G. tuberculifemur. From these results, two major conclusions have been reached:
- G. tuberculifemur may have difficulties establishing in areas where G. ashmeadi is present;
- The potential impact of releasing G. tuberculifemur in California may be negligible unless G. tuberculifemur performs better under field conditions not examined in these studies, or can fulfill a niche in the field which is not currently dominated by G. ashmeadi.
In the past, some natural enemies were deliberately chosen for biological control programs against arthropod pests because they were not highly host-specific and could maintain high populations on alternative hosts in the absence of the target pest. More recently, however, there has been a marked shift towards using highly host-specific natural enemies in biological control programs to reduce risks to non-target species, and increase the effectiveness of control (Barratt et al. 2010). New association biological control of H. vitripennis using G. tuberculifemur raises concerns about potential unwanted impacts on native non-target species of sharp shooters. It has been suggested by Simberloff and Stiling (1996) that if a natural enemy and pest have not co-evolved, non-target species are likely to be affected at least as much as the target. In no-choice trials, G. tuberculifemur successfully parasitized eggs of two native US non-target sharpshooter species, H. liturata (Ball) and Oncometopia sp. (both Cicadellinae: Proconiini) (Jones et al., 2005a). These laboratory results, along with demonstrations of successful parasitization of at least five species of Argentinean Cicadellini sharp shooters in the field suggest that G. tuberculifemur is polyphagous, and may successfully exploit native non-target sharpshooter species in the tribes Proconiini and Cicadellini. Choice and no-choice assay designs would need to be conducted to determine if similar levels of polyphagy would be observed in areas where releases of G. tuberculifemur would be considered (i.e.: California) or unintended incursion could occur (the Southeast US and Northeast Mexico, where H. vitripennis is native and sympatric with other sharpshooter species).
Authorization for the release of G. tuberculifemur in California has received preliminary approval, pending final confirmation (CDFA, 2005; D.J.W. Morgan, pers. comm.). At this time, April 2011, no releases have been made.
Gonatocerus deleoni
In 2002, Gonatocerus tuberculifemur was imported into quarantine at UC Riverside from San Rafael, Argentina. It was later discovered that these parasitoids from Argentina, originally believed to be a single-species sample, actually contained a second distinct species, which was later named Gonatocerus deleoni. Originally referred to as G. tuberculifemur “Clade 2” (Triapitsyn et al., 2008), G. deleoni is genetically and morphologically distinct from G. tuberculifemur. Like G. tuberculifemur, G. deleoni has no evolutionary history with H. vitripennis and if released into California, this would make G. deleoni a “new association” biological control agent of H. vitripennis.
The CDFA anticipated that G. deleoni would prove to be a more successful biological control agent than G. tuberculifemur because the host and geographic range of G. deleoni is narrower than G. tuberculifemur (Triapitsyn et al. 2008). In Mendoza Province, Argentina, G. deleoni has only been reared from sharpshooters in desert areas which have high climatic similarity to California. The climate in these areas differs greatly to that of the southeastern USA where H. vitripennis is native (Jones 2003). Theoretically, G. deleoni should not be able flourish in the southeastern USA which could minimize establishment risks and reduced threats of attacks to non-target native leafhoppers in this area should G. deleoni be accidentally introduced here from California (de León et al. 2008).
Quarantine studies were performed to determine G. deleoni’s viability and potential to be introduced into California as a biological control agent. Trials were conducted to compare egg age preference, competitive ability, and behavior between G. deleoni and G. ashmeadi, the dominant parasitoid of California. When searching concurrently for Homalodisca vitripennis egg masses, G. ashmeadi consistently outperformed G. deleoni by parasitizing 59-89% more eggs under three different experimental systems in the laboratory with varying host densities, egg ages, and exposure times (Irvin and Hoddle, unpublished data). G. ashmeadi had a significantly female biased sex ratio for all three experimental designs, whereas, G. deleoni offspring sex ratio was not significantly greater than 50%. A female biased sex ratio is a favorable attribute because faster parasitoid population growth results which may increase the likelihood of pest control and greater numerical domination by this parasitoid.
In contrast to predictions, parasitism rates of G. deleoni on H. vitripennis eggs was 42% lower than those demonstrated by G. tuberculifemur (Irvin and Hoddle, 2010; Irvin and Hoddle, unpublished data). This may indicate that G. deleoni is less efficient at parasitizing H. vitripennis compared to G. tuberculifemur, possibly due to it being more host specific (Triapitsyn et al. 2008).
Based on results from these quarantine studies, we speculate that: (1) G. deleoni may have difficulties establishing in areas where G. ashmeadi is present, and (2) the potential impact of releasing G. deleoni in California may be negligible unless G. deleoni can occupy and provide substantial benefit a niche in the field not currently occupied by G. ashmeadi.
Anagrus epos
Anagrus epos Girault (Hymenoptera: Mymaridae) is a common and widespread egg parasitoid of leafhoppers (Cicadellidae) in North America. A. epos is commonly collected as a parasitoid of grape leafhopper (Erythroneura spp.), but in 2004 it was collected from eggs of Cuerna fenestella Hamilton (Hemiptera: Cicadellidae), a native proconiine sharpshooter in Minnesota (Hoddle and Triapitsyn 2004). This was the first time A. epos was collected from a sharpshooter species. Although A. epos has no evolutionary history with H. vitripennis, the Minnesota strain was introduced into California in 2005, making it a “new association” biological control agent of H. vitripennis in California. A. epos was thought to be a particularly promising biological control agent of GWSS due to its gregarious nature (producing up to 12 offspring per GWSS egg) and its ability to overwinter. Additionally, it parasitizes six other species of cicadelid in California (Krugner et al. 2008) so was expected to reproduce and proliferate at times of the year when GWSS eggs are not present. It was hoped that these attributes would enable A. epos to survive the winter and reproduce in large numbers to parasitize the first generation of GWSS eggs when Gonatocerus egg mass parasitism is generally low.
Production and release of A. epos was discontinued in 2009 after no recoveries were made from GWSS eggs collected from field release sites (D. Morgan, CDFA, pers. comm.). Reasons for poor recovery may include: A) poor survival of A. epos in the field [A. epos failed to complete development from egg to adult at 35.9oC in the laboratory; Krugner et al. 2009]; B) no successful parasitism of GWSS eggs occurred in the field after release; C) difficulty of maintaining field-collected egg masses sufficiently long enough to allow emergence of A. epos [development from egg to adult takes approx. 30 days at 24oC; Krugner et al. 2009]; or D) competition from G. ashmeadi simply prevent A. epos establishing.
Non-Target Impact Studies |
Biological control has come under increased scrutiny because there is some evidence that under certain circumstances some natural enemies released for the control of a pest species may attack non-target species and adversely affect the populations of these organisms (Hoddle 2004b). To assist in the prediction and minimization of unwanted environmental effects that may be associated with exotic natural enemies released for the control of GWSS, native California sharpshooters have been studied to see if they are vulnerable to attack by parasitoids native to the southeastern USA, northeastern Mexico, and Argentina.
| Figure 9. A comparison between the smoke tree sharpshooter and GWSS. |
In California, non-target species that are closely related to GWSS include the proconiine sharpshooters H. insolita (Walker) and H. liturata Ball (smoke-tree sharpshooter) ), four sharpshooters of the cicadellini tribe, Colladonus montanus (Van Duzee) (Cherry mountain leafhopper), Graphocephala atropunctata (Signoret) (blue-green sharpshooter), Draeculacephala minerva Ball (green sharpshooter) and Xyphon fulgida (Nottingham) (red-headed sharpshooter), and other species of leafhoppers from a different subfamily Euscelidius variegatus (Kirschbaum), and Macrosteles fascifrons (Stål) .
Boyd and Hoddle (2007) conducted choice and no-choice studies in the laboratory to investigate non-target impacts on H. liturata, G. atropunctata and D. minerva from G. ashmeadi and G. fasciatus. The only non-target species susceptible to G. ashmeadi was H. liturata. H. liturata and D. minerva were parasitized by G. fasciatus under a small scale Petri dish environment, thereby both proving to be physiological acceptable hosts for G. fasciatus. However, only H. liturata was an ecologically acceptable host when given the opportunity to attack hosts on larger ‘whole plants’.
Eggs of thirteen test species were screened for their suitability as hosts for Anagrus epos in the laboratory including eight species of cicadelid, two species of Coleoptera and three species of Lepidoptera. In addition to GWSS, A. epos successfully completed development in the eggs of six other cicadelid species tested, H. liturata, G. atropunctata, Amblysellus grex (Orman), Erythroneura variabilis Beamer (variegated leafhopper), Macrosteles severini, and Circulifer tenellus (Baker) (beet leafhopper) (Krugner et al. 2008). The eighth species of cicadelid tested, Siphanta acuta (Walker) (the torpedo bug) was not a suitable host. Although A. epos appeared to parasitize eggs of the two beetle species, Phoracantha recurva Newman and P. semipunctata (F.) (eucalyptus long-horned borers), parasitized eggs failed to produce A. epos offspring. The three Lepidoptera species tested, Heliothis virescens (F.) (tobacco budworm), Ephestia kuehniella Zeller (Pyralidae) (Mediterranean flour moth) and Grapholita molesta (Busck) (oriental fruit moth) were not suitable hosts for A. epos.
| Figure 10. Blue-green sharpshooter. |
| Figure 11. A blue-green sharpshooter on citrus foliage. |
In no-choice studies, G. tuberculifemur successfully parasitized eggs of two native US non-target sharpshooter species, H. liturata Ball and Oncometopia sp. (both Cicadellinae: Proconiini) (Jones et al., 2005a). Investigation of the field host range in Argentina demonstrated that G. tuberculifemur successfully parasitized at least five species of Cicadellini, a tribe to which H. vitripennis does not belong (Jones et al., 2005b). G. deleoni appears to be highly host specific in Argentina as it has only been recorded attacking Tapajosa rubromarginata (Signoret) (Cicadellidae) and not eggs of Cicadellini (Triapitsyn et al. 2008).
Homalodisca liturata, a native sharpshooter from the same tribe and genus and most similar to GWSS (Figures 10 and 11) in its egg laying and generalist plant feeding habits, is expected to be utilized by introduced Gonatocerus species for the classical biological control of H. vitripennis. The habitats occupied by three other native sharpshooters, however, have less overlap with GWSS in addition to being from a different tribe. The habitats of the cicadelline sharpshooters X. fulgida, and the green sharpshooter, Draeculocephala minerva Ball, both consist of grasses such as Bermuda and Johnson grass, Cynodon dactylon (L.) and Sorghum halapense (L.) respectively. The bluegreen sharpshooter (Figures 12 and 13), G. atropunctata, also in the cicadellini tribe, prefers humid, partially shaded and densely vegetated habitats. This sharpshooter is often found in coastal or riparian habitats consisting of trees, vines and succulent shrubs. The different habitats occupied by these three non-target sharpshooter species, different egg laying habits (e.g., small eggs laid singly or small eggs laid in groups), and the absence of any records indicating emergence of non-native Gonatocerus species from eggs of these species may, in combination, make these non-target sharpshooter species improbable alternate hosts for Gonatocerus spp. parasitoids used for the classical biological control of GWSS. Additionally, sticky cards and malaise trap surverys in southern Californian habitats occupied by G. atropunctata have yielded no captures of the widespread and established parasitoid G. ashmeadi. This suggests that even though G. atropunctata and G. ashmeadi are sympatric, this parasitoid does not forage in detectable densities in habitats preferred by this non-target species. Because native sharpshooter eggs are approximately one-half the size of GWSS eggs, are often laid singly, and embedded into stem material rather than in groups just below the lower leaf surface as is characteristic of GWSS egg masses (Boyd and Hoddle 2007), make these native non-target sharpshooters in California unlikely hosts for GWSS parasitoids.
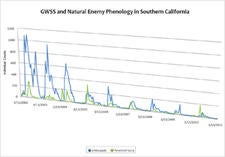
Natural Enemy Phenology in Southern California |
Sampling of GWSS populations every two weeks in organic lemons grown at the University of California Riverside Agricultural Operations Facility (UCR AgOps) has shown that the densities of this pest have declined dramatically in southern California over the last 8.5 years. In 2010, average peak population densities of GWSS were only ~7% of what was measured in 2002, indicating that populations of this pest have declined by around 93% at this study site. The most probable cause for GWSS population declines are natural enemies, in particular egg parasitoids, with G. ashmeadi being the major contributor to GWSS suppression at UCR Ag. Ops. These bi-weekly surveys have documented that natural enemies provide, on average, year round around egg parasitism of ~25% which may have been sufficient to cause the observed declines in GWSS densities. Long-term monitoring of GWSS at UCR Ag Ops is ongoing and the exact mechanisms behind these observed declines are still to be elucidated
Biology of GWSS Egg Parasitoids
Mymarid (Hymenoptera: Mymaridae) parasitoids which attack GWSS eggs are small, approximately 0.5 – 1.5 mm (0.02 – 0.06 inches) in length. Parasitoid larvae pupate within GWSS eggs and then chew circular holes through which they emerge in search of mates and new host eggs to attack. Gonatocerus ashmeadi, G. morrilli and G. triguttatus are solitary endoparasitoids that lay one egg into each individual GWSS egg within an egg mass. Gonatocerus fasciatus is gregarious, and females deposit two, rarely three eggs per GWSS egg, yielding multiple parasitoid offspring per host egg (Triapitsyn 2006).
| Figure 12. A parasitized GWSS egg mass on citrus foliage. |
The density of searching female parasitoids has a significant effect on the sex ratio of progeny produced. When female Gonatocerus parasitoids fail to encounter other ovipositing females on a GWSS egg mass, progeny output is strongly female-biased. Irvin and Hoddle(2006) demonstrated that approximately 1 male : 8 females, are produced for G. ashmeadi, 1 : 14 for G. triguttatus, and 1 : 9 and for G. fasciatus when these parasitoids don’t encounter other females of the same species trying to parasitize eggs in the same egg mass. Increasing the number of conspecific ovipositing females from one to two individuals per egg mass significantly reduced percentage of female offspring by up to 15.0% for all three Gonatocerus species. These results suggest that local mate competition affects progeny production because more males are produced when females encounter conspecifics and this results in more sons to mate with the daughters produced by competing females. The net result of higher male production when several females are foraging on the same egg mass is a greater genetic contribution by females whose sons mate with more daughters. This would not be achieved if more daughters than sons were produced.
No-choice laboratory studies showed progeny production for G. ashmeadi, G. triguttatus, and G. fasciatus was greatest from GWSS eggs 3, 4 and 2 days of age, respectively (Irvin and Hoddle, 2005). Furthermore, each parasitoid species was able to utilize a range of egg ages around their most preferred age, these being eggs 1 - 4, 3 - 6, and 1 - 3 days of age for G. ashmeadi, G. triguttatus and G. fasciatus, respectively. Parasitized GWSS eggs 8 to 10 days of age produced few parasitoid progeny. This most likely occurred because of the advanced stage of development of the GWSS embryo and parasitoids that did emerge had been oviposited into sterile or dead host eggs lacking a GWSS embryo (Irvin and Hoddle 2004). However, when given a choice between GWSS eggs 1, 3, and 5 days of age presented simultaneously to G. ashmeadi and G. triguttatus, no oviposition preferences were observed (Irvin and Hoddle 2005). This suggests these two parasitoids will attack host eggs without preference as long as eggs are of a suitable age for oviposition. The choice studies indicated G. fasciatus had preference for GWSS eggs one and three days of age while eggs five days of age were not utilized. The small size of G. fasciatus in comparison to G. ashmeadi and G. fasciatus possibly limits the range of GWSS egg ages available for parasitism. This may occur because the smaller ovipositor of G. fasciatus may be unable to pierce the chorion of older eggs as they harden during maturation.
Biology of Gonatocerus ashmeadi. Gonatocerus ashmeadi is a self-introduced exotic species which has been present in California since 1978 (Huber, 1988). Genetic studies indicate this parasitoid is native to the southeast United States and probably invaded California with H. vitripennis (Vickerman et al., 2004). G. ashmeadi is a solitary endoparasitoid that parasitizes H. vitripennis eggs and is the key natural enemy of H. vitripennis egg masses in California, providing around 12% and 19% parasitism of spring and summer H. vitripennis generations, respectively (Pilkington et al., 2005).
A significant amount of laboratory work has been conducted to define basic aspects of the reproductive (Irvin and Hoddle, 2005a, b; Irvin et al., 2006, 2007; Irvin and Hoddle, 2006, 2007), and developmental biology (Pilkington and Hoddle, 2006; Chen et al., 2006a), and behavior (Velema et al., 2005; Chen et al., 2006b) of G. ashmeadi. Female G. ashmeadi do not feed on their hosts, and display no preoviposition periods (Chen et al., 2006a). Although G. ashmeadi shares these characteristics with pro-ovigenic species (i.e., species that are born with a full complement of eggs at birth and do not mature more eggs as they are oviposited [Jervis et al., 2001]), evidence of oosorption and the process of egg maturation demonstrated in recent laboratory studies suggest that G. ashmeadi is a syn-ovigenic species which can mature more eggs in excess of those females are initially born with (Irvin and Hoddle, 2009). Other trials have shown that additional supposedly pro-ovigenic mymarids species can mature additional eggs after emergence from hosts, resulting in conclusions that these species, too, exhibit synovigeny (Carbone and Rivera, 2003; Riddick, 2005b).
G. ashmeadi reproduces through a process called arrhenotoky (Triapitsyn et al., 2003), where female offspring arise from fertilized diploid eggs, and haploid males arise from unfertilized eggs (Flanders, 1965). Results from laboratory studies have indicated that the percentage of male progeny produced by female G. ashmeadi generally increases from a minimum of 8% to a maximum of 100% as females age from 1-14 days (Irvin and Hoddle 2007). This suggests that, under the conditions of these studies, females may run out sperm with which to fertilize eggs.
It has been demonstrated in laboratory studies that there is a positive correlation between size of female G. ashmeadi and initial egg load at time of birth (i.e., females < 12 hrs of age (Irvin and Hoddle 2009). Prior studies on other species of parasitoids have shown that larger female parasitoids are more reproductively successful in the field than smaller females (Kazmer and Luck, 1995; Ellers et al., 1998) and they also experience reduced risk from environmental challenges because they are more robust (Bartlett, 1962).
Enhancing GWSS Parasitoid Survival and Parasitism Rates in the Field |
Conservation biological control has been defined as ‘modification of the environment or existing practices to protect and enhance specific natural enemies of other organisms to reduce the effect of pests’ (Eilenberg et al 2001). Conservation biological control is affected by either (1) habitation manipulation to improve natural enemy fitness and effectiveness; or (2) reducing mortality of natural enemies caused by pesticide use. Habitation manipulation often involves increasing species diversity and structural complexity of agro-ecosytems to provide resources such as shelter, pollen and nectar, and/or alternative prey and alternative hosts for natural enemies (Gurr et al. 2004). Some examples of conservation biological control include the provision of grassy ridges called ‘beetle banks’ in cereal crops, sowing flowering plants underneath orchards and vineyards, the use of sown wild-flower strips, intercropping two food crops, and reducing pesticide use to conserve natural enemies.
In the laboratory, it has been shown that floral, extrafloral nectar, and honeydew can maximize parasitoid longevity, fecundity, searching activity, and parasitism rates and female sex ratios (Tylianakis et al., 2004; Berndt and Wratten, 2005; Irvin et al., 2006b). However, herbicide and insecticide use in citrus orchards (preferred GWSS breeding and overwintering areas) and vineyards and extreme orchard hygiene can remove potential floral resources (e.g., weeds) that would provide food for parasitoids. Food resources that have shown the most potential for enhancing parasitoid populations in agricultural areas include buckwheat (Fagopyrum esculentum Moench) (Tylianakis et al., 2004; Irvin et al., 2006b), alyssum (Lobularia maritima L.) (Chaney, 1998; Irvin et al., 2006b), and insect honeydew (Johnson and Stafford, 1985; Miller, 1989).
Irvin et al. (2007) demonstrated that honey-water and buckwheat significantly increased the longevity of both male and female G. ashmeadi, G. triguttatus, and G. fasciatus up to 1760%, 1223%, 1359%, respectively when compared with individuals given only water . Survival of female G. ashmeadi provided citrus foliage infested with soft scale (Coccus hesperidum L. [a common citrus pest]) was 565% higher than parasitoids on citrus foliage alone, and alyssum increased female survival 252% compared with water.
Further studies investigating the effect of food sources on fitness of female G. ashmeadi showed that access to softscale honeydew, honey-water, alyssum, and buckwheat flowers resulted in up to 378% more male progeny, 198% more female progeny, and 273% total progeny than those females provisioned with water only (Irvin and Hoddle 2007). There was no significant difference in progeny production or survival between alyssum and buckwheat, and while the overall number of progeny was greater in any given food treatment than for water alone, the overall mean progeny did not significantly differ between those food treatments other than water.
The fact that all food treatments significantly increased female G. ashmeadi longevity and fecundity indicates that resource procurement is not only essential for enhancing G. ashmeadi survival, but is also important for increasing G. ashmeadi fecundity. This is not only due to increased longevity allowing females more time to parasitize hosts, but also shows a direct increase in fecundity due to improved nutrition.
Understorey management (i.e., the deliberate management of flowering plants beneath orchards and vineyards) is potentially one way to enhance parasitoid populations in agricultural systems thereby leading to improved pest control by natural enemies (Landis et al. 2000). Sowing flowering plants [e.g., buckwheat, dill (Anethum graveolens L.), or alyssum as an understory in citrus orchards or vineyards harboring H. vitripennis could potentially provide a food source to Gonatocerus species. It is hoped that the use of these understorey plants will enhance biological control of H. vitripennis in California by increasing longevity which may improve host and mate encounter rates, and enhancing fecundity which could result in a higher proportion of H. vitripennis eggs being parasitized.
A series of studies were conducted to investigate the strengths and limitations of cover cropping under the unique growing conditions representative of grape producing areas of Southern California. This four-year project was funded by Western Region of Sustainable Agriculture Research and Education (Western SARE) and began in June 2007. The project investigated the use of buckwheat (Fagopyrum esculentum Moench) and cahaba vetch (Vicia sativa L.) for management of arthropod grape pests in California and included a large-scale field trial located at Bella Vista vineyard in Temecula, California. Preliminary results suggest that cover cropping may not prove to be a viable option for grape growers in southern California due to the difficulty of establishing cover crops in southern California climate, cost of irrigation water, and both cover crops testing positive for harboring X. fastidiosa. Additionally, results from sticky trap and visual count data showed that additional irrigation required by the cover crop may lead to increased populations of pestiferous leafhoppers and other pests. These studies demonstrate that understorey management may only be a suitable option for enhancing natural enemies in climates where flowering plants can be established naturally without supplemental irrigation and pest densities are not inadvertently increased as a result of this practice.
The Invasive Potential of the Glassy-winged Sharpshooter |
GWSS has shown strong invasive potential having established outside of its home range in California [established ca.1990 (Sorensen and Gill 1996)], French Polynesia [established 1999 (Cheou 2002)], Hawaii [established 2004 (Hoover 2004)], Easter Island [established 2005 (Sandra Ide pers. comm.)], and the Cook Islands [established 2007 (Disna Gunawardana pers. comm.)].Modeling that combines regional climate data and relevant biological information has indicated California’s premier wine growing areas of Napa, Sonoma, and Mendocino counties may be vulnerable to invasion by GWSS while states north of California, also with substantial grape industries, may be too cold to harbor permanent populations of GWSS (Hoddle 2004a). The major wine-growing regions of New Zealand, Australia, the Bordeaux region of France, most areas of Spain, as well as central and southern Italy have climates that could be conducive to GWSS establishment and proliferation should it be accidentally introduced (Hoddle 2004a).
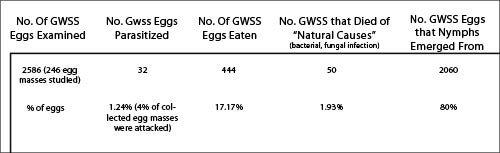
GWSS established in Tahiti French Polynesia in 1999 and was likely introduced accidentally on ornamental plants imported from California. Tahiti is the largest and most populous island in the group of islands collectively known as the Windward Islands in the Society Islands Archipelago in French Polynesia in the South Pacific. French Polynesia is very remote; it is 6,000 km west of Chile, and 5,200 km east of Australia. The Cook Islands lie 900 km to the west of French Polynesia and Easter Island is 5,178 km to the east. GWSS flourished in Tahiti because of an extremely mild year round climate and an abundant supply of exotic and ornamental plants maintained in gardens. In contrast to California, where there are just two generations [spring and summer] each year), natural enemies are lacking, and no obvious competitors exist in urban or natural settings, GWSS populations underwent exponential growth. At this time naturally occurring parasitism of GWSS eggs was very low on the island of Mo’orea, the immediate neighboring island to Tahiti. Surveys indicated that less than 2% of individual eggs were attacked by generalist egg parasitoids in just 4% of egg masses collected. Of those egg masses attacked, 44% of the eggs were parasitized. Because only a few eggs in an egg mass were attacked (indicating inefficient and opportunistic exploitation) and only males were reared from GWSS eggs (which suggest poor host quality as females did not oviposit fertilized female eggs), and the wasp responsible for attacking GWSS egg masses was a species of platygasterid, a family that does not specialize on sharpshooter eggs, but will parasitize various species of leafhoppers, indicated there were no native specialized parasitoids attacking GWSS. Consequently, GWSS populations in French Polynesia were free of the pressures associated with natural enemies and this pest reached extraordinarily high densities in Tahiti. Watery excreta known as sharpshooter rain, literally rained from infested trees because there were so many GWSS feeding on trees. Such high populations retarded plant growth and reduced local fruit production. Further, the use of shade trees was reduced because so much GWSS rain made them unsuitable for escaping the intense tropical sun. Finally, at night, incredible numbers of GWSS would be attracted to lights and large numbers would fly into homes, shops, and business at night. This was very annoying because adult GWSS would die and need to be swept up, the wingbeat frequency of adults flying past heads caused an unpleasant “buzz” in ears, and adults would occasionally “bite” people when they landed on exposed skin and probed with their needle-like mouthparts.
To combat the GWSS infestation in French Polynesia it was decided that the best strategy to use would be classical biological control. To this end, the mymarid egg parasitoid Gonatocerus ashmeadi was imported into quarantine in Tahiti in September 2004 and studied to make sure it did not pose any undue risk to other insects already present in French Polynesia. Following these studies, the accumulated evidence strongly indicated that the parasitoid was likely safe for release and posed no undue risk to non-target species in French Polynesia. The parasitoid was released on May 2, 2005 on Tahiti.Because of high levels of human activity and interisland movement of plants between Tahiti and other islands in French Polynesia, GWSS was moved rapidly to other island groups in French Polynesia. In 2001, it was found in Raiatea (Leeward Islands) and in 2002 in Moorea. Populations were then discovered in the Leeward Islands of Huahine and Bora Bora in 2003, and in Tahaa and Maupiti in 2005. At the end of 2004 and the beginning of 2005, GWSS populations were discovered outside of the Society Islands in two other archipelagos of French Polynesia substantially distant from Tahiti: the Australs, where two islands were infested (Rurutu and Tubuai) (January 2005) and the Marquesas, where one island, Nuku Hiva, was found infested in November 2004.
Between May and October 2005, 13,786 parasitoids were released at 27 sites in Tahiti. The parasitoid established readily, and within seven months of release GWSS had been completely controlled in Tahiti and GWSS populations were reduced by over 95%. The parasitoid also spread unassisted to every other island infested by GWSS. This rapid movement between islands strongly suggested that quarantines that were established to reduce GWSS were not working and people were still moving plants infested with GWSS eggs. However, this time eggs were parasitized by G. ashmeadi, and this is how it was moved unintentionally between islands and archipelagos.
Additional information. For more information on the biological control efforts of GWSS in Tahiti and French Polynesia, you can download this free fact sheet.
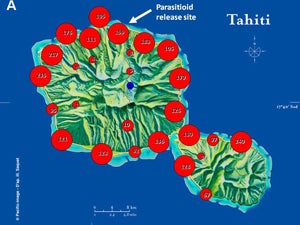
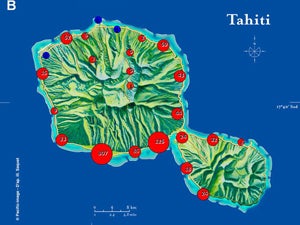
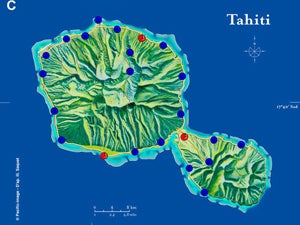
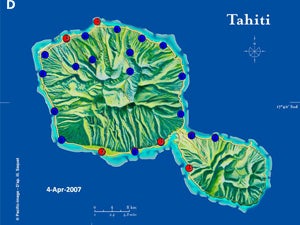
| Map 1. Reduction of GWSS populations on Tahiti following the release of G. ashmeadi. (A) GWSS densities on Tahtiti from one minute sweep net sampling of hisbiscus bushes before the release of G. ashmeadi on May 2 2005; (B) GWSS densities five months (October 1 2005) after parasitoid release; (C) GWSS densities seven months after parasitoid release (December 12 2005); (D) GWSS densities two years after the release of G. ashmeadi (Figure courtesy of J. Grandgirard and J. Petit.). | |||
References |
- Barratt, B.I.P., Howarth, F.G., Withers, T.M., Kean, J.M., Ridley, G.S., 2010. Progress in risk assessment for classical biological control. Biological Control 52: 245–254.
- Bartlett, B. R. 1962. The ingest ion of dry sugars by adult entomophagous insects and the use of this feeding habit for measuring the moisture needs of parasites. Journal of Economic Entomology 55 (5): 749-753.
- Berndt, Lisa A., Wratten, Steve D. 2005. Effects of alyssum flowers on the longevity, fecundity, and sex ratio of the leafroller parasitoid Dolichogenidea tasmanica. Biological Control 32 (1): 65-69.
- Boyd, E. A., Hoddle, M.S. 2007. Host specificity testing of Gonatocerus spp. egg-parasitoids used in a classical biological control program against Homalodisca vitripennis: A retrospective analysis for non-target impacts in southern California. Biological Control 43: 56-70.
- Carbone, S. S., Rivera, A. C. 2003. Egg load and adaptive superparsitism in Anaphes nitens, an egg parasitoid of the Eucalyptus snout-beetle Gonipterus scutellatus. Entomologia Experimentalis et Applicata 106: 127–134.
- CDFA. 2003. Pierce's disease control program - report to the legislature, May 2003 [Online]. Available at: http://www.cdfa.ca.gov/phpps/pdcp/docs/2002LegReport.pdf. (verified 8 July 2004)
- CDFA, 2008. Pierce’s Disease Program Annual Report to the Legislature 2008. California Department of Food and Agriculture, Sacramento, CA, USA.
- Chaney, W.E. 1998. Biological control of aphids in lettuce crops using in-Weld insectaries. In: Pickett, C.H., Bugg, R.L. (Eds.), Enhancing Biological Control—Habitat Management to Promote Natural Enemies of Agricultural Pests. University of California Press, Berkeley, CA, pp. 73–83.
- Chen, Wen-Long, Leopold, Roger A., Morgan, David J. W., et al. 2006a. Development and reproduction of the egg parasitoid, Gonatocerus ashmeadi Girault (Hymenoptera : Mymaridae), as a function of temperature. Environmental Entomology 35 (5): 1178-1187.
- Chen, W. L., Leopold, R. A., Harris, M. O. 2006b. Parasitism of the glassy-winged sharpshooter, Homalodisca coagulata (Homoptera : Cicadellidae): Functional response and superparasitism by Gonatocerus ashmeadi (Hymenoptera : Mymaridae). Biological Control 37 (1): 119-129.
- Cheou D. 2002. Incursion of glassy-winged sharpshooter Homalodisca coagulata in French Polynesia. Plant Protection Service Pest Alert:1.
- Costa H.S., Blua, M.J., Bethke, J.A., Redak, R.A. 2000. Transmission of Xylella fasitidiosa to oleander by the glassy-winged sharpshooter, Homalodisca coagulata. HortScience 35(7):1265-1267.
- De León, J.H., Logarzo, G.A., and Triapitsyn, S.V. (2008), Molecular characterization of Gonatocerus tuberculifemur (Ogloblin) (Hymenoptera: Mymaridae), a prospective Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae) biological control candidate agent from South America: divergent clades. Bulletin of Entomological Research 98: 97-108.
- de Lima, J. E. O., Miranda, V. S., Hartung, J. S., Brlansky, R. H., Coutinho, A., Roberto, S. R., Carlos, E. F., 1998. Coffee leaf scorch bacterium: Axenic culture, pathogenicity, and comparison with Xylella fastidosa of citrus. Plant Disease 82: 94-97.
- Eilenburg, J., Hajek, A., Lomer, C., 2001. Suggestions for unifying the terminology in biological control. Biocontrol 46: 387-400.
- Ellers, Jacintha, Van Alphen, Jacques J. M., Sevenster, Jan G. 1998. A field study of size-fitness relationships in the parasitoid Asobara tabida. Journal of Animal Ecology 67 (2): 318-324.
- Esser, T. West, D. (eds.), 2010. Pierce’s Disease Control Symposium Proceedings, December 15-17, San Diego, California, pp. 283. California Department of Food and Agriculture, Sacramento, CA. Available from: http://www.cdfa.ca.gov/pdcp/2010_Research_Proceedings.html.
- Flanders, Stanley E. 1965. On the sexuality and sex ratios of hymenopterous populations. American Naturalist 99 (909): 489-494.
- Gurr, G. M., Scarratt, S. L., Wratten, S. D., Berndt, L., Irvin, N. 2004. Ecological engineering, habitat manipulation and pest management. In: Gurr, G. M., Wratten, S. D., Altieri, M. A. (Eds.), Ecological Engineering for Pest Management: Advances in Habitat Manipulation for Arthropods, CSIRO Publishing, Collingwood, VIC, Australia, pp. 1-12.
- Gurr G.M., Wratten, S.D., Luna, J.M. 2003. Multi-function agricultural biodiversity: Pest management and other benefits. Basic & Applied Ecology 4(2):107-16.
- Hartung, J. S., Beretta, J., Brlansky, R. H., Spisso, J., Lee, R. F., 1994. Citrus variegated chlorosis bacterium: Axenic culture, pathogenicity, and serological relationships with other strains of Xylella fastidiosa. Phytopathology 84: 591-597.
- Hernandez-Martinez, Rufina, de la Cerda, Karla A., Costa, Heather S., et al. 2007. Phylogenetic relationships of Xylella fastidiosa strains isolated from landscape ornamentals in southern California. Phytopathology 97 (7): 857-864.
- Hoddle M.S. 2004a. The potential adventive geographic range of glassy-winged sharpshooter, Homalodisca coagulata and the grape pathogen Xylella fastidiosa: Implications for California and other grape growing regions of the world. Crop Protection 23 (8): 691-699.
- Hoddle M.S. 2004b. Restoring balance: Using exotic natural enemies to control invasive exotic species. Conservation Biology 18 (1): 38-49.
- Hoddle, M. S., Triapitsyn, S. V., 2004. Searching for and collecting egg parasitoids of the glassy-winged sharpshooter in the central and eastern USA. In: Tariq, M. A., Oswalt, S., Blincoe, P., Ba, A., Lorick, T., Esser, T. (Eds.), Proceedings of the Pierce’s Disease Research Symposium. California Department of Food and Agriculture, 7-10 December 200, San Diego, CA., pp. 342-344.
- Hokkanen, H.M.T., and Pimentel, D. 1989. New associations in biological control: theory and practice. Canadian Entomologist 121: 829-840.
- Hoover W. 2004. New invader may threaten crops. The Honolulu Advertiser, May 14, 2004, Honolulu.
- Huber, J. T. 1988. The species groups of Gonatocerus Nees in North America with a revision of the Sulphuripes and Ater groups (Hymenoptera: Mymaridae). Memoirs of the Entomological Society of Canada 141: 109.
- Irvin, Nicola A., Hoddle, Mark S. 2004. Oviposition preference of Homalodisca coagulata for two Citrus limon cultivars and influence of host plant on parasitism by Gonatocerus ashmeadi and G. triguttatus (Hymenoptera:Mymaridae). Florida Entomologist 87 (4): 504-510.
- Irvin N., Hoddle, M.S. 2005a. Determination of Homalodisca coagulata (Hemiptera: Cicadellidae) egg ages that are suitable for oviposition by Gonatocerus ashmeadi, G. triguttatus and G. fasciatus (Hymenoptera: Mymaridae): (1) no choice tests. Biological Control 32 (3): 391-400.
- Irvin, N. A., Hoddle, M. S. 2005b. The competitive ability of three mymarid egg parasitoids (Gonatocerus spp.) for glassy-winged sharptshooter (Homalodisica coagulata) eggs. Biological Control 34 (2): 204-214.
- Irvin, N. A., Hoddle, M. S. 2006. The effect of intraspecific competition on progeny sex ratio in Gonatocerus spp. for Homalodisca coagulata egg masses: Economic implications for mass rearing and biological control. Biological Control 39 (2): 169-170.
- Irvin, Nicola A., Hoddle, Mark S. 2007. Evaluation of floral resources for enhancement of fitness of Gonatocerus ashmeadi, an egg parasitoid of the glassy-winged sharpshooter, Homalodisca vitripennis. Biological Control 40 (1): 80-88.
- Irvin, Nicola A., Hoddle, Mark S. 2009. Egg maturation, oosorption, and wing wear in Gonatocerus ashmeadi (Hymenoptera: Mymaridae), an egg parasitoid of the glassy-winged sharpshooter, Homalodisca vitripennis (Hemiptera: Cicadellidae). Biological Control 48 (2): 125-132.
- Irvin, N. A., Hoddle, M. S. 2010. Comparative assessments of Gonatocerus ashmeadi and the 'new association' parasitoid Gonatocerus tuberculifemur (Hymenoptera: Mymaridae) as biological control agents of Homalodisca vitripennis (Hemiptera: Cicadellidae). Biological Control 55 (3): 186-196.
- Irvin, Nicola A., Hoddle, Mark S., Castle, Steven J. 2007. The effect of resource provisioning and sugar composition of foods on longevity of three Gonatocerus spp., egg parasitoids of Homalodisca vitripennis. Biological Control 40 (1): 69-79.
- Irvin, N. A., Hoddle, M. S., Morgan, D. J. W. 2006. Competition between Gonatocerus ashmeadi and G. triguttatus for glassy-winged sharpshooter (Homalodisca coagulata) egg masses. Biocontrol Science and Technology 16 (4): 359-375.
- Irvin, N.A., Hoddle, M.S., Suarez-Espinoza, J., 2009. The functional response of Gonatocerus ashmeadi and the ‘new association’ parasitoid G. tuberculifemur attacking eggs of Homalodisca vitripennis. Environmental Entomology 38 (6): 1634–1641.
- Jervis, Mark A., Heimpel, George E., Ferns, Peter N., et al. 2001. Life-history strategies in parasitoid wasps: A comparative analysis of 'ovigeny'. Journal of Animal Ecology 70 (3): 442-458.
- Johnson, J. B., Stafford, M. P. 1985. Adult Noctuidae feeding on aphid honeydew and a discussion of honeydew feeding by adult Lepidoptera. Journal of the Lepidopterists' Society 39 (4): 321-327.
- Jones, W. A., Logarzo, G. A., Triapitsyn, S.V., Casas, M., Virla, E. G., Purcell, A.H., 2005a. Biology and host range of two South American egg parasitoids (Hymenoptera: Mymaridae), possible biocontrol agents for glassy-winged sharpshooter (Say) (Hemiptera: Cicadellidae: Proconiini). Proceedings of the 12th International Auchenorrhyncha Congress and 6th International Workshop on Leafhoppers and Planthoppers of Economic Significance, University of California, Berkeley, 7-12 August 2005, Berkeley, CA, Available online at http://nature.berkeley.edu/hoppercongress/
- Jones, W. A., Logarzo, G. A., Virla, E. G., Luft, E., 2005b. Environmental risk assessment of egg parasitoids from South America: non-target field and laboratory host range in Argentina and the U. S. In: Tariq, M. A., Blincoe, P., Mochel, M., Oswalt, S., Esser, T. (Eds.), Proceedings of the Pierce’s Disease Research Symposium. California Department of Food and Agriculture, 5-7 December 2005, San Diego, CA, pp. 343-344.
- Kazmer, David J., Luck, Robert F. 1995. Field tests of the size-fitness hypothesis in the egg parasitoid Trichogramma pretiosum. Ecology 76 (2): 412-425.
- Krugner, R., Johnson, M. W., Groves, R. L., Morse, J. G. 2008. Host specificity of Anagrus epos: a potential biological control agent of Homalodisca vitripennis. Biocontrol 56: 439-449.
- Krugner, R., Johnseon, M. W., Morgan, D. J. W., Morse, J. G. 2009. Production of Anagrus epos Girault (Hymenoptera: Mymaridae) on Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae) eggs. Biological Control 51: 122-129.
- Landis D.A., Wratten, S.D., Gurr, G.M. 2000. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annual Review of Entomology 45:175-201.
- Messenger, P.S., and van der Bosch, R. (1971), The adaptability of introduced biological control agents. In: Huffaker, C.F. (ed.), Biological Control, Plenum Press: New York, pp. 68-92.
- Miller, W. E. 1989. Reproductive enhancement by adult feeding effects of honeydew in imbibed water on spruce budworm. Journal of the Lepidopterists' Society 43 (3): 167-177.
- Montero-Astua, M., Saborio, G. R., Chacon-Diaz, C., Garita, L., Villalobos, W., Hartung, J. S., Rivera, C., 2008. First report of Xylela fastidosa in Avocado in Costa Rica. Plant Disease 92 (1): 175.
- Pilkington, L.J., Irvin, N.A., Boyd, E.A., Hoddle, M.S., Triapitsyn, S.V., Carey, B.G., Jones, W.A., Morgan, D.J.W., 2005. Introduced parasitic wasps could control glassy-winged sharpshooter. California Agriculture 59 (4): 223–228.
- Pilkington, Leigh J., Hoddle, Mark S. 2006. Reproductive and developmental biology of Gonatocerus ashmeadi (Hymenoptera : Mymaridae), an egg parasitoid of Homalodisca coagulata (Hemiptera : Cicadellidae). Biological Control 37 (3): 266-275.
- Pimentel, D. 1963. Introducing parasites and predators to control native pests. Canadian Entomologist 95: 785-792.
- Riddick, E. W. 2005. Are lab-cultured Anaphes iole females strictly proovigenic? BioControl 50 (6): 911-919.
- Salt, G., 1961. Competition among insect parasitoids: mechanisms in biological competition. Symposium of the Society for Experimental Biology 15: 96-119.
- Siebert J., 2001. Economic impact of Pierce's disease on the California grape industry. Pierce's Disease Research Symposium: 111-116.
- Simberloff, D., Stiling, P., 1996. How risky is biological control? Ecology 77 (7): 1965–1974.
- Sorensen J.T., Gill, R.J. 1996. A range extension of Homalodisca coagulata (Say) (Hemiptera: Clypeorrhyncha: Cicadellidae) to southern California. Pan-Pacific Entomologist 72 (3):160-1.
- Triapitysn, S. V. 2006. A key to the Mymaridae (Hymenoptera) egg parasitoids of proconiine sharpshooters (Hemiptera: Cicadellidae) in the Nearctic region, with description of two new species of Gonatocerus. Zootaxa 1202:1-38.
- Triapitsyn, S.V., Logarzo, G.A., De León, J.H., Virla, E.G., 2008. A new Gonatocerus (Hymenoptera: Mymaridae) from Argentina, with taxonomic notes and molecular data on the G. tuberculifemur species complex. Zootaxa 1949: 1–29.
- Triapitsyn S.V., Morgan, D.J.W., Hoddle, M.S., Berezovskiy, V.V. 2003. Observations on the biology of Gonatocerus fasciatus Girault (Hymenoptera : Mymaridae), egg parasitoid of Homalodisca coagulata (Say) and Oncometopia orbona (Fabricius) (Hemiptera : Clypeorrhyncha : Cicadellidae). Pan-Pacific Entomologist 79 (1):75-76.
- Triapitsyn S.V., Phillips, P.A. 2000. First record of Gonatocerus triguttatus (Hymenoptera : Mymaridae) from eggs of Homalodisca coagulata (Homoptera : Cicadellidae) with notes on the distribution of the host. Florida Entomologist 83 (2): 200-203.
- Velema, H.P., Hemerik, L., Hoddle, M.S., and Luck, R.F. (2005). Brochosome influence on parasitisation efficiency of Homalodisca coagulata (Say) (Hemiptera : Cicadellidae) egg masses by Gonatocerus ashmeadi Girault (Hymenoptera : Mymaridae). Ecological Entomology 30: 485-496.
- Vickerman D.B., Hoddle, M.S., Triapitysn, S.V., Stouthamer, R. 2004. Species identity of geographically distinct populations of the glassy-winged sharpshooter parasitoid Gonatocerus ashmeadi: Morphology, DNA sequences and reproductive compatibility. Biological Control 31 (3): 338-345.
- Zwolfer, H., 1971. The structure and effect of parasite complexes attacking phytophagous host insects. In: denBoer, P. J., Gradwell, G. R. (Eds.), Dynamics of Populations: Proceedings of the Advanced Study Institute on Dynamics and Numbers in Populations. Oosterbeck 1970. Centre for Agricultural Publicating and Documentation, Wageningen, The Netherlands, pp. 405-418.