A Critical Analysis of the Extinction of Levuana iridescens in Fiji by Bessa remota
Prepared by Mark Hoddle, Extension Specialist and Director of Center for Invasive Species Research
mark.hoddle@ucr.edu
Introduction |
Non-target extinctions by natural enemies. The hypothesis that arthropod natural enemies can cause the extinction of target and non-target arthropods has been widely accepted despite a lack of plausible scientific evidence supporting extinction claims (Lynch and Thomas 2000; Lynch et al. 2001). In the environmental and ecological sciences unverified statements can reduce scientific credibility and have adverse consequences on garnering public support - both emotional and financial - for proposed future research. Such an undesirable effect is particularly pertinent for programs investigating the benefits and risks of biological control, especially when the risk of extinction, an irreversible ecological calamity, forms the central tenet of debate.
| Figure 1. Levuana iridescens |
| Figure 2. Besa remota, a natural enemy which contributed to the extinction of L. iridescens in Fiji. |
Deliberate introductions into new areas of novel organisms such as biological control agents by its very nature entail some level of risk. Risk in this instance, is the uncertainty that damage to non-target organisms could be caused by introduced natural enemies. Risk assessment estimates how much damage can result from an event involving the agent and risk management seeks to determine the acceptability of collateral damage the agent causes and devises measures to mitigate it (Lonsdale et al. 2001). Evaluation of techniques for assessing risk to non-target organisms posed by natural enemies being considered for use in biological control programs has received much research attention (Lonsdale et al., 2001) and interest in this area is continuing to grow rapidly, especially for arthropod biological control agents (Hopper 2001; Van Driesche and Reardon 2004).
Perceived risk associated with biological control projects can be amplified by invoking uncertainty that is associated with the project or practice in question (Pidgeon et al. 2003). In the case of classical biological control, risk to the ecosystem receiving a novel exotic natural enemy pertains to unpredictable, unintended, and disruptive trophic perturbations that could be manifested once an exotic natural enemy permanently establishes in the receiving area (Louda and Stiling 2004). Such unintended consequences, should they occur, can be used to disparage the science underlying risk assessment in biological control because the environmental insult, should it occur, is irreversible, and the outcomes should have been predictable and avoided if the science underlying risk assessment is solid (Stiling and Simberloff 2000).
Extinction is the most easily understood of all ecological phenomena and species imperilment figures prominently in daily news and lay publications. Extinction events are unflattering commentaries about human stewardship of ecosystems, especially when deliberate actions with the aim of improving ecosystem health, such as management of invasive species with natural enemies, can be correlated with extinction events. Moral indignation about extinction is particularly high when attractive or rare and endangered organisms or habitat have been extirpated by deliberately introduced biological control agents.
One of the most famous and "best documented cases of extinction" in biological control is the extirpation of the pestiferous levuana moth, Levuana iridescens Bethune-Baker (Lepidoptera: Zygaenidae) in Fiji, by the deliberately introduced parasitic fly, Bessa (Ptychomyia) remota (Aldrich) (Diptera: Tachinidae), from Malaya (Howarth, 1991; 2001; New 2005). Levuana iridescens is only known from Fiji, and the last authentic specimen of this attractive "endemic" monotypic zygaenid genus was apparently collected in Fiji in 1929 (Howarth, 1991). Bessa remota has also been assumed to have caused the extinction of another endemic Fijian zygaenid, Heteropan dolensDruce (Lepidoptera: Zygaenidae) (Howarth 1991, 2001; Robinson 1975; New 2005). Recently, this untested hypothesis, that an exotic natural enemy, B. remota, is responsible for the extinction of L. iridescens has been challenged (Kuris 2003; Sands 1997), but a larger part of this story has been overlooked and deserves attention. The remainder of this article provides additional historical background on the "Levuana Campaign" not covered by the Tothill et al. (1930) treatise, further challenges the assertion by Howarth (1991, 2001) that extinction of L. iridescens and H. dolens has occurred, and presents an update on recent museum, library, and field surveys for L. iridescens in Fiji.
A Synopsis of Levuana iridescens in Fiji
| Figure 3. A palm tree destroyed by L. iridescens. |
Levuana iridescens was first recorded as a serious coconut pest around 1877 from a single island, Viti Levu, in the Fijian archipelago, a collection of approximately 300 islands. Earlier records on coconut production from 1846 and 1860 do not indicate a severe and widespread malady affecting palms (Simmonds, 1924). On Viti Levu, the only location this moth is known from, outbreaks of this moth were frequent and devastated coconut palms as moth larvae trenched undersides of leaves that often led to defoliation and palm mortality. As a consequence, copra production (i.e., dried coconut meat from which coconut oil is extracted) was severely affected on Viti Levu and coconut production was unprofitable and indigenous Fijian culture, which relied on the coconut for food, water, fiber, medicinal products, fuel, and building materials was adversely affected (Tothill et al., 1930). Since its first detection, L. iridescens was restricted to Viti Levu for approximately 40 years before it began expanding its range in 1916 to close offshore islands where conditions favored pest establishment and proliferation.
The highly restricted geographic range of L. iridescens was considered a "fact contrary to the usual position with regard to the endemic fauna of Fiji" (Simmonds, 1924). Scientists devising management schemes for L. iridescens concluded that the pest was not endemic to Fiji and was an exotic invader (Simmonds, 1921a; 1924). This conclusion was arrived at because L. iridescens exhibited frequent outbreaks, was expanding its geographic range, and lacked specialized parasitoids associated with eggs, larvae, or pupae. These facts were recognized as very peculiar aspects of this pest's ecology when compared to other zygaenid species in their native range which outbreak infrequently, don't exhibit range expansion, and have diverse suites of associated natural enemies (Simmonds, 1924; Simmonds 1930a; Tothill et al., 1930). This circumstantial evidence did not, however, prove conclusively that L. iridescens was not native to Fiji, but did strongly suggest the moth had originated elsewhere and immigrated to the islands (Tothill et al., 1930). Unless L. iridescens is found outside of Fiji the tautology of this endemicity argument is difficult to conclusively resolve.
| Figure 4. J.D. Tothill (center) and his two associates, R.W. Paine (left) and C. Widley (right) at Lunga, Solomons (1928). |
To curb the spread and impact of L. iridescens in Fiji and limit the threat to other coconut growing nations in the South Pacific the "Levuana Campaign" was initiated in 1925 after a £5,000 reward failed to conjure a magical solution to the problem. The leader of the campaign, J.D. Tothill, and his two associates T.H.C. Taylor and R.W. Paine were given a two year contract to resolve the problem. Tothill viewed biological control as the only feasible and sustainable option available for permanently suppressing L. iridescens. Tothill et al. (1930) located and imported a tachinid fly, B. remota, from Malaya where it controlled another palm defoiliating zygaenind, Artona catoxantha. Within six months of release of this fly from quarantine in August-September 1925, L. iridescens populations had been reduced to almost non-detectable levels on Viti Levu, although persistent outbreaks continued on two small off shore islands (Nukulau and Makuluva) in the Rewa River Delta (Tothill et al., 1930). The last specimen of L. iridescens was apparently collected in 1929 and is now assumed to be extinct because of B. remota (Howarth 2001).
Kuris (2003) reviewed Tothill et al.'s (1930) treatise on the classical biological control of L. iridescens in Fiji with B. remota from the perspective of an invasion biologist. Kuris (2003) concluded that it was highly probable that L. iridescens was exotic to Fiji and that it was unlikely that natural enemies, in particular, B. remota, had caused the extinction of L. iridescens. Kuris's (2003) analysis supports earlier statements and conclusions reached by Sands (1997) regarding the exotic origin and extinction of L. iridescens. Interestingly, a much larger part of the L. iridescens story has been overlooked. Biological control was the last control option turned to after other management strategies had failed.
Pre-1925 Management Strategies for Levuana iridescens in Fiji |
Cultural control strategies. Cultural controls are techniques that are developed from crop management or mechanical practices that can be readily manipulated to disadvantage pest population growth while having no adverse effect on crop health. Cultural control practices can include the use of plant varieties resistant to pest attack, soil amendments to improve plant health and vigor, the removal of alternate food sources the pest utilizes, or the use of traps to capture the target pest thereby reducing its prevalence in a localized area.
Host plant resistance. Oviri coconuts from Tahiti were tested for resistance to feeding by L. iridescens larvae. The astringent properties of this coconut variety were presumed to act as potential deterrents to herbivory and lack of obvious insect attack on coconuts in Tahiti provided anecdotal field evidence suggesting that Oviri coconuts may be unpalatable to phytophagous insects (Simmonds 1920). Importation of Oviri coconuts into Fiji and subsequent feeding trials assessing palatability to emergent and half-grown L. iridescens larvae were inconclusive leading to the decision that high yielding resistant coconut varieties were not likely to be found easily (Simmonds 1921a).
Plantation management. To reduce the debilitating impact of L. iridescens outbreaks on palm growth, practices designed to promote plant vigor to enable infested palms to grow through defoliation events were promoted. Outreach programs encouraged plantation managers to control weeds, thin overcrowded plantations, use soil amendments, and to drain swampy ground (Tothill 1926). There are no data indicating the success of these suggested practices, or the extent to which they were employed for L. iridescens control.
Traps. Light traps set at night were assessed for their ability to attract adult L. iridescens. Since this moth is a day-flying insect, powerful acetylene lamps set over pans of kerosene mixed with water failed to attract moths at night (Knowles 1919). Adult moths had been observed feeding at a variety of different flowers including Lantana camara L., coconut, mango (Mangifera indica L.), ginger (Zingiber spp.), mile-a-minute (Polygonum perfoliatum L., and Tournefortia argenta (Simmonds, 1925; Tothill et al., 1930). Oddly, phenol and tar also had alluring properties (Simmonds 1925). Knowles (1919) speculated that if attractive volatile additives could be identified and isolated they could be used increase the attractiveness of traps to adult moths thereby enhancing the utility of this method of control. There is no published research assessing the feasibility of using traps for monitoringL. iridescens populations, determining pest phenology, or implementation for localized control efforts.
| Figure 5. Vulu thatch on a coconut palm tree. |
Vulu stripping. Vulu is the thatch or fiber matting that connects the bases of coconut fronds. Levuana iridescens larvae preferentially pupate in vulu. During Levuana outbreaks, densities of pupating larvae in vulu could become so great that cocoons would be spun over the top of existing cocoons rending emergence impossible for larvae in lower strata (Tothill et al., 1930). Manual removal of vulu as a pest suppression tactic was considered, but quickly abandoned due to the intensive amount of labor required, and the difficulty of quickly ascending and descending tall coconut palms. Additionally, vulu removal destroyed habitat used by beneficial arthropods such as spiders that preyed upon pests (Knowles 1909).
Host plant eradication. In addition to coconut, L. iridescens larvae feed on several other palm species, including Actinophloeus macarthuri, Areca catechu (betel nut), Elueis guineensis, Guilelma speciosa, Livistona chinensis and L. speciosa, Oreodoxa regia and O. oleracea(royal palms), Sabal palmetto, Sagus vitiensis (native sago palm), and Veitchia joannis (niu sawa). During severe outbreaks, high densities of L. iridescens would "spill over" onto less preferred hosts and eggs and feeding larvae would be found on Artocarpus incisa (breadfruit), bananas, reeds, sugar cane, and unidentified orchids (Tothill et al. 1930; Simmonds 1925). "Drastic steps" such as the removal of food sources "to starve out" L. iridescens were considered necessary in combination with burning and arsenical sprays to eradicate L. iridescens on recently invaded islands that lay offshore from Viti Levu (Simmonds 1922). A similar project proposed for Viti Levu would have eradicated all preferred hosts, in particular, coconuts and royal palms. The enormity of the project, the cost, and the uncertainty of eliminating every host plant led to the abandonment of this strategy at an early stage of planning (Simmonds 1922; 1924).
Quarantine inspections to curb spread. In 1924, sea ports at Suva, Levuka (all coconuts within 0.5 miles of this wharf were destroyed), and Lautoka were designated as inspection ports under the Diseases of Plants Ordinance (1913) and inter-island cutters were required to stop for inspection before moving from infested zones to non-infested zones (Tothill et al. 1930). A tripartite cooperative involving growers, The Native Department, and the Government oversaw the inspection process. Vessels without special exemptions (some passenger, plantation owned, and freight boats were not legally required to stop) were subjected to inspection at one of the three ports and were awarded an Inspection Certificate and entry records were gazetted. Vessel inspections were voluntarily overseen by growers who had the authority to destroy all "objectionable and dangerous material" on boats moving between islands (Tothill 1925a). Objectionable and dangerous material of primary concern was the movement of vulu between islands. Vulu, the fibrous material that grows at the base of coconut palm fronds, was regularly harvested and used as packing material, for wrapping perishable (e.g., taro roots and medicines) and fragile items, and enclosing letters Tothill 1925a). Vulu is the preferred pupation site for L. iridescens larvae, and pupae on vulu can survive without sustenance for up to nine days making human mediated long distance transport of this life stage plausible (Tothill 1925a). The inspection system remained in place until 1928, "by which time Levuana moth had been reduced to a condition of impotency" by B. remota (Tothill et al. 1930).
Chemcial and Biological Control Approaches |
The need to quickly control outbreaks of L. iridescens and for eradication of incipient populations of this pest as it expanded its range to islands surrounding Viti Levu prompted investigation of insecticides as a control strategy. Efficacy of stomach poisons applied as foliar sprays mixed with seawater were assessed over the period 1909 - 1925. insecticides were evaluated with seawater as the carrier because seawater is more readily available than freshwater in Fiji (Knowles 1909) and coconuts have a high tolerance to being drenched with seawater (Tothill et al. 1930). One pound of arsenic boiled with soda and mixed with 400 gallons of sea water, starch (as a sticker), and whiting (for coloration) readily killed L. iridescens larvae (Knowles 1919). Alternatively, 1.5 lbs of either lead or calcium arsenate mixed with 40 gallons of seawater killed larvae within 5-10 days, and provided control for up to two months (Tothill 1925b). Approximately 12-24 months suppression was achieved with 2.5 lbs of dry lead arsenate mixed with 40 imperial gallons of seawater when applied to the undersides of coconut leaves. Paris green was unsuitable because of phytotoxicity and poor adhesion to coconut leaves (Tothill et al. 1930).
Application of sprays to tall palms involved either the use of a power-driven pump or the erection of scaffolding around each palm to be treated by hand. A major shortcoming associated with power application of wet sprays to tall coconut palms was the enormity of wasted spray to provide adequate coverage of infested coconut crowns. Costs associated with the employment of spray crews (Knowles 1919) and scaffold erection and movement were prohibitively expensive for hand applications that treated palm crowns at close range (Knowles 1919). Small coconut palms were successfully hand-treated for L. iridescens with kerosene emulsions or resin washes (Froggatt 1914) and applications of 30 oz of lead arsenate in 25 gallons of water applied at a rate of 4 gallons per palm with applications repeated at 5 week intervals also controlled larvae on small palms (Jepson 1915).
| Figure 6. A cloud of sulfur fumigation in a coconut plantation. |
A rapid reaction spray rig was custom developed for deployment against incipient L. iridescens populations on previously uninfested islands. This spray rig consisted of a sea-going barge with a motorized Fitz-Henry Guptill solid stream sprayer attached to its deck. A galvanized iron structure was built to cover the pump to protect both it and the crew from adverse weather and excessive sun exposure. The barge was towed by a launch along coastlines and spray hoses up to 0.5 miles long could be dragged into plantations needing treatment. The barge had ready access to seawater for mixing with poisons and beach access overcame difficulties of getting spray equipment into areas that lacked roads (Tothill 1925c). Trees 60-80 feet tall could be treated when weather conditions were appropriate and coconuts in close proximity to beaches and within drag line distance could be treated with this spray rig. The rapid reactionary capability of the floating spray rig was never tested as B. remota rendered obsolete the need for insecticidal controls and treatment of new infestations on previously uninfested islands (Tothill et al. 1930).
Fumigation of infested coconut plantations was also investigated. Sulfur mixed with tar was turned over dry coconut leaves. This resulted in a thick smoke that would waft through infested plantations. Unless L. iridescens larvae were actually scorched by the flames they suffered no mortality. Clouds of smoke in plantations were never dense enough to suffocate adult moths, but did succeed in agitating adult moths thereby encouraging flight and dispersal (Knowles 1919).
Biological Control. Levuana iridescens was not considered native to Fiji and its home range was presumed to lie to the north-west of Fiji (Tothill et al., 1930). The assumption for this geographic area of origin was that from 1802 onwards a vigorous trade in sandalwood was routed from Cochin China through Fiji and the New Hebrides (i.e., Vanuatu) to areas east of Fiji. From 1864 onwards, extensive recruitment of labor for Fijian plantations from the Solomons and Vanuatu occurred and this was considered another possible area from which L. iridescens may have originated (Simmonds, 1924). Extensive foreign exploration by Simmonds (1924) for approximately nine months (June 14, 1922 - February 11, 1924) of coconut palms and native palms such as sago, was conducted throughout New Guinea, Vanuatu, Bismarcks, and Solomons. Specifically, Simmonds (1924) searched the northwest coast of New Guinea, New Britain, Witu, New Ireland, Bouganville, Shortlands, Russell Group, New Georgea, Gela, Ysabel, Guadacanal, Malaita, Manning Straits, Banks Group, Epi, Santos, Malakula, Pau Uma, Aoba, Penticoste, Sandwich, and Tanna. This search failed to locate L.iridescens (Simmonds, 1924). Concurrently, A.M. Lea of the Adelaide Museum (Australia) surveyed northern Queensland, Thursday Island group, and Magentic Island. Lea's efforts in 1924 also failed to locate L. iridescens (Despeissis, 1925). Consequently, L. iridescens is only known from Fiji.
| Figure 7. Cages containing Besa remota for release at Caboni. |
Lea then focused efforts in the Malay archipelago where he was assisted by G.H. Corbett, B.A.R. Gater, and S. Leefmans in the search for another zygaenid, Artona catoxantha, that exhibited periodic outbreaks resulting in defoliated palms reminiscent of damage caused by L. iridescens. Artona catoxantha was known to have suite of natural enemies that effectively regulated its population growth. It was speculated that some of these natural enemies should they be located may attack L. iridescens as larvae of these two moths exhibited similar host plant associations, biology, ecology, and feeding behavior. Attempts by Lea and others to successfully ship live A. catoxantha parasitoids from the Federated Malay States to Fiji prior to 1925 failed. In 1925, H.W. Simmonds was stationed in Kuala Lumpur to await outbreaks of A. catoxantha and if possible to serendipitously coordinate shipments of parasitoids collected from unpredictable outbreaks located somewhere in the Federated Malay States with infrequent (approximately 1-2 per year) sea-going freighters leaving this area en route to Fiji. Airplanes were unavailable and the cost of chartering a naval or merchant vessel for the 4,000 mile journey was prohibitive (Tothill et al. 1930). T.H.C. Taylor was dispatched by Tothill in 1925 to re-explore areas of New Guinea and then to push onto Cochin China. During this sojourn Taylor stopped to visit Simmonds and this trip coincided with an A. catoxantha outbreak at Batu Gajah, 175 km north of Kuala Lumpur.
Parasitized and unparasitized larvae (20,000 in total) were collected, caged on 85 potted palms, transported 300 miles by train to Singapore where the cargo was loaded on the Clan MacKay for Surabaya Java on July 10, 1925. The cargo was transferred to Clan Matheson on July 12, 1925 and this ship arrived in Suva, Fiji on August 3, 1925 with 315 live adult B. remota- 25 days after leaving Batu Gajah (Tothill et al., 1930). The suitability of L. iridescens as a host was unknown until the parasitoids arrived in Fiji and were presented with L. iridescens larvae in quarantine. In quarantine, adult flies immediately parasitized L. iridescens and fly larvae developed successfully on L. iridescens. Hyperparasitoids were removed from the L. iridescens colony. By January 1926, 32,621 flies had been successfully reared on L. iridescens and liberated. Complete control of L. iridescens was achieved approximately six months after releases of B. remota commenced (Tothill et al., 1930) and is cited as a premier example of classical biological control (Caltagirone 1981).
Evaluation of the Koronivia Research Station Records |
| Figure 8. Levuana collection at the Koronivia Research Station. |
Howarth's (1991; 2001) claim that L. iridescens was extinct by 1929, but may have persisted until the 1950's and that the endemic H. dolensis extinct in Fiji (Robinson 1975) was investigated by searching library accessible literature records and Fijian insect collections. In October 2002 and 2004, the author examined the insect collection at the Koronivia Research Station near Suva on Viti Levu, Fiji, for L. iridescens and H. dolens. The Koronivia collection has 14 specimens of adult L. iridescens; eight specimens have collection data, the remaining six are unlabeled. There are two L .iridescens cocoon samples on vulu, one is a single cocoon and the other is a mass of approximately 20 cocoons. One sample of larval L. iridescens trenching on the underside of a coconut leaf approximately 5 cm x 1.3 cm in size with around eight trenches has been preserved. The trenched leaf was collected in Serea, Viti Levu, April 13, 1939, the collector is not named. The most recently lodged specimens of adult L. iridescens were collected in December 1953, at Taulevu (due west of Vunindawa) on Viti Levu. Larvae were also collected from this L. iridescens outbreak in Taulevu that affected 100 coconut palms. The larvae were not located in the Koronivia collection. The most recently deposited specimens of Heteropan dolens are two adults collected from Taulevu in 1963. Detailed collection data for L. iridescens and H. dolens are provided. In October 2004, a large number of unlabeled Schmidt Boxes and Cornell Drawers were inspected in the Koronivia Collection for “undiscovered” L. iridescens and B. remota specimens. The search yielded six additional L. iridescens, and one cocoon sample. All of this “new” material was unlabeled so collection date and locality are unknown. Two of the best unlabeled adult L. iridescens specimens were returned to the Entomology Museum at the University of California at Riverside for long-term curation. The overall condition of the specimens in the Koronivia Collection is extremely bad and further deterioration will occur without immediate efforts aimed at preservation.
Published outbreak records for L. iridescens post-1925 housed in library collections were searched for in the using Entomology Abstracts. Accessible citations that could be located were used to construct a map documenting the location and year of the published outbreak. The last published journal record was 1942 (17 years post-release of B. remota) and the final mention in the literature of L. iridescens being observed on Viti Levu was 1956 (31 years post-release) (Paine 1994).
Foreign Exploration and Future Research |
MSH and DPA Sands (CSIRO Indooroopilly, Australia) attempted to locate L. iridescens on Viti Levu over the period October 29, 2002 - November 6, 2002 in southeastern Viti Levu around Vunindawa, Colo-i-Suva, and Laucala Point. The search for larvae and adult moths followed two concurrent avenues: (1) visual inspection of palm fronds for larvae and coconut flowers for adult moths; (2) broadcast distribution of "wanted posters" with colored images of L. iridescens adults, larvae, and trenched leaves to village residents, and (3) sweep netting flowers (e.g., lantana) within coconut plantations. The poster images were taken from the Tothill et al. (1930) treatise and $100 (US) bounty was offered for the successful capture of any life stage of L. iridescens. The poster campaign was unsuccessful; no Lepidoptera were turned in for identification.
| Figure 9. Levuana "wanted posters" were distributed to village residents. |
Tothill et al. (1930) state clearly that visitors intending to witness L.iridescens damage to coconuts post-release of B. remota should visit two islands in the Rewa River Delta, Nukulau and Makuluva. Bessa remota had failed to provide consistent control of L. iridescens on these small isolated islands and it was assumed that a lack of secondary hosts for B. remota to sustain itself during periods of low L. iridescens density was the major cause for this lack of persistent suppression (Tothill et al. 1930). A similar situation with respect to secondary hosts and primary pest suppression currently exists on Cape Cod, Massachusetts U.S.A. Brown tail moth, Euproctis chrysorrhoea (Lepidpotera: Lymantridae) a serious forestry pest, is successfully suppressed by the tachinid C. concinnata in more interior areas but E. chrysorrhoea is unregulated in areas of Cape Cod where secondary hosts are unavailable to maintain high populations of C. concinnata (J. Elkinton pers. comm. 2004).
Attempts by MSH and DPAS to gain access to Nukulau and Makuluva by motor boat were thwarted due to a heavy military presence and successful interception on the beach of Nukulau. Both islands have been converted to prison camps to house militants responsible for the attempted May 19, 2000 coup d’etat lead by Fijian Nationalist George Speight and public access is prohibited. Further, the floral diversity of these two islands appears to have increased from Tothill et al.'s (1930) time. From the sea, visual observation suggested that the remnants of old coconut plantations were suffering from lack of human management as thick understoreys were evident. These prevailing conditions would most likely create habitat that would favor increased lepidopteran biodiversity, which may be conducive to sustaining B. remota populations leading to permanent suppression of L. iridescens on small off shore islands.
During an outbreak, L. iridescens exhibits a clear attack sequence. It preferentially attacks the tallest coconut palms in a highly localized area. Once these palms are defoliated, surrounding palms are then attacked until the lowest growing palms are infested last (Knowles 1919). Based on this description of the outbreak ecology of L. iridescens, and the fact that severe and prolonged outbreaks no longer occur, it is most likely that this insect inhabits the tallest palm trees in areas that support small populations. Consequently, visual searches for L. iridescens larvae on small immature coconut palms in southeastern Viti Levu were unsuccessful in 2002.
With this attack sequence in mind, aerial malaise traps were deployed at Serea, Taulevu, Vunindawa, and Toga Island near Nausori on the Rewa River Delta over the period October 5–31, 2004. Traps were suspended on ropes 20-25m above the ground between adjacent coconut palms and immediately under the palm crown. Traps were lowered on a rope pulley system and collection bottles with 95% ethanol were checked every 3-4 days for L. iridescens, H. dolens, and B. remota over a four week period. None of the target species were collected.
| Figure 10. Damage to palm fronds caused by A. argaula. |
Visual searches of palm fronds for L. iridescens larvae were greatly hindered by feeding damage caused by larvae of another frond trenching lepidopteran, the coconut flat moth, Agonoxena argaula Meyrick (Agonoxenidae). This pest causes damage to undersides of palm fronds identical to L. iridescens. In direct contrast to L. iridescens, A. argaula larvae spin silk roofs over the open tops of trenches they inhabit. Abandoned trenches lack silk coverings and are impossible to distinguish from those made by L. iridescens larvae. Additionally, A. argaula larvae drop to the ground to pupate and they do not utilize vulu as a pupation site. Agonoxena argaula was present in Fiji during the "Levuana Campaign" but was apparently insignificant (Simmonds 1921b; Tothill et al. 1930). Population densities of A. argaula may have increased substantially following the successful biological control of L. iridescens as competition for undersides of palm fronds would have been greatly diminished. The high population densities of A. argaula and the common occurrence of severe feeding damage on palms would indicate that at the time surveys for L. iridescens were conducted, coconut flat moth was not being efficiently regulated by natural enemies.
Future Research
The creditability of Howarth's (1991; 2001) assertion that biological control of L. iridescens with B. remota in Fiji is one of the "best documented cases of extinction" has been strongly challenged by invasion biologists (Kuris 2003) and biological control specialists (Sands 1997). Data supporting Howarth's claims that natural enemies have driven L. iridescens to extinction are non-existent or at best must be considered weak as no comprehensive and prolonged campaigns to search for this zygaenid have been undertaken to verify the continued presence of L. iridescens in Fiji. Unfortunately, the published extinction assumption of L. iridescens and H. dolens by B. remota has largely been accepted as fact (New 2005) despite a lack of confirmatory evidence (Lynch et al 2001), museum records to the contrary, and published reports of persistent L. iridescens outbreaks up to 1956. Post 1956, research by entomologists employed by Imperial Bureau of Entomology (a.k.a. Imperial Commonwealth International Institute of Entomology) to service tropical countries in the British Colonial Territories in the South Pacific began to wane and research on Fijian coconut problems diminished. By 1966, the post-war Pacific Island projects were ended and research teams returned to the United Kingdom (Paine 1994). The reduced presence of skilled entomological observers working on coconuts (a crop of declining economic value) in Fiji most likely accounts for the cessation of reports of L. iridescens outbreaks and also the lack of observations on B. remota which has not been seen since the last observation of L. iridescens in 1956.
The endemicity of L. iridescens to Fiji is unresolved but has been vigorously questioned by Sands (1997) and Kuris(2003). If L. iridescens is native to Fiji why did persistent outbreaks begin on Viti Levu around 1877? It is possible that new varieties of coconuts that were being grown in large plantations and the associated habitat modification allowed L. iridescens to completely escape any regulatory effect of it’s suite of endemic natural enemies. Further, this creation of natural enemy free space via agricultural practices inexplicably extended to encompass a number of native Fijian palm species and other unrelated crop plants (e.g., bananas and bread fruit). This natural enemy-free space was initially restricted exclusively to Viti Levu before undetermined factors facilitated its extension onto nearby islands in 1916. This scenario while interesting, is implausible and makes it more likely that L. iridescens is exotic to Fiji as it exhibited persistent population outbreaks and continued to spread into nearby and previously uninfested islands. Today, these phenomena are recognized to represent scenarios typical of invasive species infiltrating new areas.
The claim that H. dolens is extinct in Fiji and B. remota is responsible (Howarth 2001; New 2005; Robinson 1975) is particularly worrisome. There are no published field or laboratory data that H. dolens is attacked by B. remota(Tothill, et al. 1930), that larvae are suitable hosts for B. remota on which to complete development, or that this fly forages in habitat or on host plants used by this zygaenid. Further, H. dolens is known to exist on other Pacific Islands (e.g., Aneityum Island, Vanuatu [Robinson 1975]). Unverified extinction claims in the literature have undoubtedly amplified the social perception of risk when conservation biologists and ecologists suggest extinction of non-target species by biological control agents is well documented and L. iridescens is used as the example (Howarth 1991; 2001).
It is very difficult to prove that the relatively recent absence of a species is due to its extinction. Zygaenids that are well regulated by natural enemies are known to exhibit long lag periods between outbreaks and this is a clearly known facet of their ecology. For example, Artona chorista was presumed to have gone extinct until an outbreak 100 years after its initial description occurred in cardamom plantations in Sikkim (Tarmann 2005).
A robust approach would be to compile available data that a species is absent and then assess whether the combined weight of that evidence is sufficient to assume that extinction has occurred. This type of analysis is advocated by the Committee on Recently Extinct Organisms (CREO 2004; Holloway 2005) and editors of peer reviewed articles should insist that these standards be reached prior to publishing statements that species extinctions have occurred. To substantiate or refute the extinction of L. iridescens by B. remota within the framework suggested by CREO, a comprehensive multi-year search for L. iridescens in Fiji should be conducted, preferably between August and December (this time of year was when outbreaks were most severe) and efforts should be concentrated in the southeastern corner of Viti Levu in the area circumscribed by Serea, Taulevu, Vunindawa, Colo-i-Suva and Laucala Point (see Map). The tallest coconut palms in these areas should form the foci of searches.
| Figure 11. L. iridescens pupal cases. |
Indirect evidence for the existence of L. iridescens could come from the discovery of pupal cases on vulu whose silk chemistry properties are identical to those of cocoons in the Koronivia Collection. Two subsamples of L. iridescens cocoons from the Koronivia Collection have been deposited in the Entomology Museum at the University of California Riverside. Very small pieces from both cocoon samples were removed from masses and subjected to analyses to determine the “protein fingerprint” of the silk. The results of the analyses indicated very little difference in the amino acid profiles for both samples indicating that they are most likely from the same species. These profiles could be used to compare “fingerprints” from silk cocoons collected from vulu in Fiji to determine if they belong to L. iridescens. Silk amino acid profiles are often very specific to particular species (C. Hayashi pers. comm. 2005) and this forensic analysis could provide strong indirect evidence for the continued existence of L. iridescens in Fiji.
Direct evidence of the presence of L. iridescens would be the capture of adult moths or larvae. Aerial malaise traps should be hung between the tallest palms at canopy level in search areas to intercept adult moths flying to feed on coconut flowers, or seeking oviposition sites, or mates. Fogging of palm canopies with insecticides to knock down foliage feeders may result in the collection of adult or larval L. iridescens, but persistent maritime breezes will make this strategy very difficult (T. Irwin pers. comm. 2004). Surveys may best be conducted after cyclones have hit the islands because of the disruptive effect major storms have on biological control agents thereby allowing pest species to temporarily escape natural enemy regulation (Paine 1931; 1994). Confirmation of the existence of L. iridescens should be followed with an intensive effort to live trap adult moths, especially females, to develop colonies.
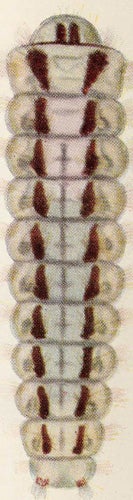
| Figure 12. Levuana iridescens larva. |
Tothill et al. (1930) speculated that pheromones played a major role in mate location by male L. iridescens. Isolation and identification of a sex pheromone would enable the development of pheromone traps that could be deployed in island groups to the west of Fiji, the presumed historical home range of L. iridescens. Use of pheromone traps would allow the efficient sampling of low density L. iridescens populations and would be an important tool in delineating the geographic range of this moth outside of Viti Levu. Pheromone traps are used routinely for the monitoring of western grapeleaf skeletonizer, Harrisina metallica Stretch (=brillans Barnes and McDunnough) (tribe = Procridini), a pestiferous zygaenid of grapes in the U.S.A. (Soderstrom et al. 1985). Blends of pheromone constituents with known attractiveness to H. metallica could be deployed in Fiji in anticipation of eliciting partial responses (cross attraction) from male L. iridescens. However, L. iridescens (tribe Artonini) may not respond to procridin pheromone blends (Gerhard Tarmann pers. comm. 2004).
Finally, the non-target impact and spread of B. remota in the Fijian islands needs research attention. The best approach to address these two issues would be through the use of food web analyses (Memmott 2000) in a manner similar to studies conducted in Hawaii to determine the magnitude of infiltration and impact on native species by the unintentional spread exotic natural enemies into natural systems (Henneman and Memmott 2001).
References |
- Bias, H.P., Vepachedu, R., Gilroy, S., Callaway, R.M., Vivanco, J.M., 2003. Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 301:1377-1380.
- Barnes, A.C., 1931. Annual Report for the Year 1930. Legislative Council, Fiji. Council Paper No. 56, Department of Agriculture, pp. 1-12.
- Barnes, A.C. 1932. Annual Report for the Year 1931. Legislative Council, Fiji. Council Paper No. 20, Department of Agriculture, pp. 1-13.
- Barron, M.C., Barlow, N.D., Wratten, S.D. 2003. Non-target parasitism of the endemic New Zealand red admiral butterfly (Bassaris gonerilla) by the introduced biocontrol agent Pteromalus puparum. Biological Control 27: 329-335.
- Benson, J., Van Driesche, R.G., Pasquale, A., Elkinton, J., 2003a. Introduced barconid parasitoids and range reduction of a native butterfly in New England. Biological Control 28: 197-213.
- Benson, J., Pasquale, A., Van Driesche, R., Elkinton, J., 2003b. Asessment of risk posed to introduced barconid wasps to Pieris virginiensis, a native woodland butterfly in New England. Biological Control 26: 83-93.
- Boettner, G.H., Elkinton, J.S., Boettner, C.J., 2000). Effects of biological control introduction on three non-target native species of saturniid moths. Conservation Biology 14: 1798-1806.
- Caltagirone, L.E., 1981. Landmark examples in classical biological control. Annual Review of Entomology 26: 213-232.
- Committee on Recently Extinct Organisms. http://creo.amnh.org/ last accessed July 30, 2005.
- Despeissis, A., 1925. Levuana moth of the coconut. Legislative Council, Fiji. Council Paper No. 44, Department of Agriculture, pp. 2-3.
- Froggatt, W.W., 1914. Pests and diseases of coconut palm. New South Wales Department of Agriculture, Sydney , Science Bulletin 2: 3-63.
- Henneman, M.L., Memmott, J., 2001. Infiltration of a Hawaiian community by introduced biological control agents. Science 293: 1314-1317.
- Hickman, B. 1997. The effects of the white butterflies (Pieris rapae) introduced parasitoid (Pteromalus puparum) on the native yellow admiral, Bassaris itea. Unpublished MSc. Thesis. University of Auckland, Auckland, New Zealand.
- Hoddle, M.S., 2004a. Biological control in support of conservation: friend or foe? In: Gordon, M.S., Bartol, S.M (Eds.), Experimental Approaches to Conservation Biology. University of California Press, Berkeley, California, pp. 202-237.
- Hoddle, M.S., 2004b. Restoring balance: using exotic species to control invasive exotic species. Conservation Biology 18: 38-49.
- Hoddle, M.S., 2004c. The strength of biological control in the battle against invasive pests: a reply. Conservation Biology 18: 61-64.
- Holloway, M., 2005. When extinct isn’t – questioning the term after a bird’s return. Scientific American 293(2): 22-23.
- Hopper, K.R., 2001. Research needs concerning non-target impacts of biological control introductions. In: Wajnberg, Scott JK, and Quimby PC. (EDs.), Evaluating Indirect Ecological Effects of Biological Control. CABI Publishing, Wallingford U.K., pp. 39-56.
- Howarth, F.G., 1991. Environmental impacts of classical biological control. Annual Review of Entomology 36: 485-509
- Howarth, F.G., 2001. Environmental issues concerning the importation of non-indigenous biological control agents. In: Lockwood, J.A., Howarth, F.G., Purcell, M.F. (Eds.), Balancing Nature: Assessing the Impact of Importing Non-Native Biological Control Agents (An International Perspective). Thomas Say Publications in Entomology: Proceedings. Entomological Society of America, Lanham, Maryland, pp.70-99.
- Jepson, F.P., 1915. Report of the Entomologist. Fiji Department of Agriculture, Annual Report for the Year 1914, Suva, pp. 17-27.
- Keane, R.M., Crawley, M.J., 2002. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology Evolution 17: 164-170
- Knowles, C.H., 1909. Insects injurious to cultivated crops. Fiji Department of Agricultural Annual Report 27: 35-39.
- Knowles, C.H., 1919. The purple leaf moth of coconuts in Fiji. Reprinted in 1924 in Agricultural Circular issued by the Department of Agriculture, Fiji 5 (1) 1-14.
- Kuris, A.M. 2003. Did biological control cause extinction of the coconut moth, Levuana iridescens, in Fiji? Biological Invasions 5: 131-141.
- Lever, R.J.A.W., 1938. Division of Entomology – Annual Report for 1937. Fiji Department of Agriculture 1: 25-27.
- Lever, R.J.A.W., 1942. Division of Entomology – Annual Report for 1941. Agricultural Journal, Fiji Department of Agriculture 13: 23-24.
- Lonsdale, M., Briese, D.T., Cullen, J.M., 2001. Risk analysis and weed biological control. In: Wajnberg, E., Scott, J.K., Quimby, P.C., (Eds.), Evaluating Indirect Ecological Effects of Biological Control. CABI Publishing, Wallingford U.K., pp. 185-210.
- Louda, S.M., Kendall, D., Connor, J., Simblerloff, D., 1997. Ecological effects of an insect introduced for the biological control of weeds. Science 277: 1088-1090.
- Louda, S.M., Stiling, P., 2004. The double-edged sword of biological control in conservation and restoration. Conservation Biology 18: 50-53.
- Lynch, L.D., Thomas, M.B., 2000. Nontarget effects in the biocontrol of insects with insects, nematodes, and microbial agents: the evidence. Biocontrol News and Information 21: 117N-130N.
- Lynch, L.D., Hokkanen, H.M.T., Babendreier, D., Bigler, F., Burgio, G., Gao, Z., Kuske, S., Loomans, A., Menzler-Hokkanen, I., Thomas, M.B., Tommasini, G., Waage, J.K., van Lenteren, J.C., Zeng, Q.Q., 2001. Insect biological control and non-target effects: a European perspective. In: Wajnberg, E., Scott, J.K., Quimby, P.C. (Eds.), Evaluating Indirect Ecological Effects of Biological Control. CABI Publishing, Wallingford U.K., pp. 99-125.
- Memmott, J., (2000) Food Webs as a Tool for Studying Nontarget Effects in Biological Control. In: Follet, P., Duan, J.J., (Eds.), Nontarget Effects of Biological Control. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 147-163.
- New, T.R., 2005 Invertebrate Conservation and Agricultural Ecosystems. Cambridge University Press, Cambridge, U.K.
- Paine, R.W., 1931. Coconut Committee – Entomological Report. Annual Bulletin of Divisional Reports. Fiji Department of Agriculture 1(1): 1-8.
- Paine, R.W., 1994. Recollections of a Pacific Entomologist 1925-1966. ACIAR Canberra, Australia.
- Pearson, D.E., Callaway, R.M., 2003. Indirect effects of host-specific biological control agents. Trends in Ecology and Evolution 18: 456-461
- Pidgeon, N., Kasperson, R.E., Slovic, P., (Eds) 2003.The Social Amplification of Risk. Cambridge University Press, Cambridge U.K.
- Pimentel, D., Lach, L., Zuniga, R., Morrison, D., 2002. Environmental and Economic Costs Associated with Non-Indigenous Species in the United States. In: Pimental D., (Ed.), Biological Invasions: Economic and Environmental Costs of Alien Plant, Animal, and Microbe Species. CRC Press Boca Raton, pp. 285-303.
- Robinson, G.S., 1975. Macrolepidoptera of Fiji and Rotuma: A Taxonomic and Biogeographic Study. E.W. Classey Ltd, Faringdon, Great Britain.
- Sands, D.P.A., 1997. The safety of biological control agents: assessing their impact on beneficial and other non-target hosts. Memoirs of the Museum of Victoria 65: 611-616.
- Shea, K., Chesson, P., 2002. Community ecology theory as a framework for biological invasions. Trends in Ecology and Evolution 17: 170-176.
- Simmonds, H.W., 1920. Report on mission to Tahiti to investigate the parasites of the coconut scale with a view to their introduction to Fiji. Monthly Agricultural Circular, Fiji Department of Agriculture 1 (7): 133-138.
- Simmonds, H.W., 1921a. Notes on Levuana iridescens. Monthly Agricultural Circular, Fiji Department of Agriculture 2 (4): 84-85.
- Simmonds, H.W., 1921b. Report on coconut districts of Vunilagi and Macuata. Monthly Agricultural Circular, Fiji Department of Agriculture 2 (3): 40-43.
- Simmonds, H.W., 1922. Spread of Levuana iridescens. Agricultural Circular, Department of Agriculture, Fiji. 3 (4): 52-53.
- Simmonds, H.W., 1924. Mission to New Guinea, Bismarcks, Solomons, and New Hebrides. Legislative Council, Fiji. Council Paper No. 2, Department of Agriculture, 11pp.
- Simmonds, H.W., 1925. Report by the acting government entomologist. Fiji Department of Agriculture Annual Report, Suva 1926 pp. 7-9.
- Simmonds, H.W., 1930a. Report by H.W. Simmonds, Government Entomologist. Legislative Council, Fiji . Council Paper No. 42, Department of Agriculture, pp. 9-10.
- Simmonds, H.W., 1930. Problems in biological control. The gap in the sequence of generations in Artona catoxantha, the coconut leaf moth of Malaya. Tropical Agriculture 7: 215-219.
- Simmonds, H.W., 1935a. Entomology Division Annual Report, 1935. Annual Bulletin of Divisional Reports. Fiji Department of Agriculture 8(1): 19-22.
- Simmonds, H.W., 1935b. Entomological Notes. Agricultural Journal (issued by the Department of Agriculture, Fiji) 8: 32.
- Simmonds, H.W., 1936. Division of Entomology Annual Report for 1936. Annual Bulletin of Divisional Reports. Fiji Department of Agriculture 1(1): 27-29.
- Soderstrom, E.L., Brandl, D.G., Myerson, J., Buttery, R.G., Mackey, B.E., 1985. Sex pheromone for attracting western grapeleaf skeletonizer (Lepidoptera: Zygaenidae). Journal of Economic Entomology 78: 799-801.
- Stiling, P., Simberloff, D., 2000. The Frequency and Strength of Nontarget effects of invertebrate biological control agents of plant pests and weeds. In: Follet, P., Duan, J.J., (Eds.), Nontarget Effects of Biological Control. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 31-43.
- Tarmann, G.M., 2005. Zyganid Moths of Australia - A Revision of the Australian Zygaenidae (Procridinae: Artonini). CSIRO Publishing Collingwood Australia.
- Torchin, M.E., Lafferty, L.D., Dobson, A.P., Mckenzie, V.J., Kuris, A.M., 2003. Introduced species and their missing parasites. Nature 421: 628-630.
- Tothill, J.D., 1925a. Report by the Acting Entomologist for the Year 1923. Levuana iridescens. Agricultural Circular, Fiji Department of Agriculture 5(2) 83-85.
- Tothill, J.D., 1925b. Levuana iridescens campaign. Legislative Council, Fiji. Council Paper No. 20.
- Tothill, J.D., 1925c. An interim report on the Levuana campaign. Colonial Office Report 3 pp.
- Tothill, J.D., 1926. Work of the coconut committee. Legislative Council, Fiji. Council Paper No. 90. Department of Agriculture, page 2.
- Tothill, J.D., 1928. Annual Report for the Year 1927. Legislative Council, Fiji. Council Paper No. 75, Department of Agriculture, pp. 1-5.
- Tothill, J.D., Taylor, T.H.C., Paine, R.W., 1930. The Coconut Moth in Fiji: a History of its Control by Means of Parasites. Imperial Bureau of Entomology, London .
- Van Driesche, R.G., Bellows, T.S., Jr. (Eds.), 1993. Steps in Classical Arthropod Biological Control. Thomas Say Publications in Entomology, Entomological Society of America, Lanham, Maryland.
- Van Driesche, R.G., Reardon, R. (Eds.), 2004. Assessing Host Ranges for Parasitoids and Predators Used for Classical Biological Control: A Guide to Best Practice. Forest Health and Technology Enterprise Team, Morgantown, West Virginia, USDA-USFS, FHTET-2004-03.