The Avocado Seed Moth, Stenoma catenifer Walsingham (Lepidoptera: Elachistidae)
Prepared by Mark Hoddle, Extension Specialist and Director of Center for Invasive Species Research
mark.hoddle@ucr.edu
Introduction |
|
|
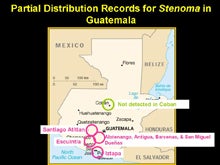
Stenoma catenifer, the avocado seed moth, is a new world species that is thought to feed exclusively on the fruit and seeds of plants in the family Lauraceae. It has been recorded feeding on fruit of avocados (Persea americana) and the greenheart tree, Chlorocardium rodiei, an important timber tree in Guyana (Cervantes Peredo et al., 1999). Stenoma catenifer is a well known avocado pest attacking fruit in Mexico, Guatemala, Costa Rica, Panama, Bolivia, Colombia, Peru, Ecuador, Venezuela, and Brazil (Acevedo et al., 1972; Boscán de Martínez and Godoy, 1984). Avocados in Guyana are likely attacked as this moth has been recorded from fruit of greenheart in this country (Cervantes Peredo et al., 1999).
Levels of S. catenifer infestation in avocados can be high. In some avocado growing regions of Brazil 100% of the crop can be infested with S. catenifer (Nava et al., 2005a), and in orchards that are sprayed with broad spectrum insecticides 7-11 times over the course of a single growing season up to 60% of fruit can be infested with larval S. catenifer (Nava et al., 2005b). Consequently, this pest is considered to be one of the major impediments to commercial avocado production in Brazil (Nava et al., 2005a,b). In Venezuela, avocado fruit infestation with S. catenifer larvae can be as high as 80% (Boscán de Martínez and Godoy, 1982).
Pest Identification and Developmental Biology |
|
Figure 2. Oviposition studies in cages indicate that up to 68% of oviposited eggs can be laid on the branch to which the avocado fruit pedicel is attached. |
|
|
Figure 3. The final fifth instar (up to 25 mm in length) is characterized by having violet dorsal coloration contrasting strikingly with a blue-turquoise colored ventral surface. |
Figure 4. The final fifth instar (up to 25 mm in length) is characterized by having violet dorsal coloration contrasting strikingly with a blue-turquoise colored ventral surface. |
|
Figure 5. Pupae are “free” chrysalises that may be loosely attached with fine weak silk strands to a substrate but can be easily dislodge with gentle prodding. |
Figure 6. Young pupae are a striking turquoise blue in color and within 4-8 hrs of initial pupation this color becomes reddish-brown as pupae mature and melonize. |
|
Figure 7. Adults moths are light tan color, and wings are marked with numerous black spots. The most characteristic marking on the forewings is the easily observable “C” shape of spots as the distal end of the forewings. |
|
Eggs and oviposition. Stenoma catenifer eggs are small and oval in shape (on average eggs are 0.59 ± 0.04 mm long and 0.38 ± 0.2 mm wide) (Cervantes Peredo et al., 1999). Eggs are laid singly and are initially white or pale cream when first laid and later darken as they mature. Female moths oviposit at night with peak oviposition occurring within four hours of darkness in which time 80% of eggs are laid (Nava et al., 2005a). Stenoma catenifer females tend to lay eggs on rough surfaces and in crevices such as the fruit pedicel or necrotic spots on fruit (Hohmann et al., 2003).
Oviposition studies in cages indicate that up to 68% of oviposited eggs can be laid on the branch to which the avocado fruit pedicel is attached (Hoddle unpublished data, 2007). In the laboratory, caged females can be induced to lay eggs on rough quilted paper towel as long as avocado fruit are provided as a stimulus to induce egg laying (Nava et al., 2005a).
Larvae. First instar larvae are pale cream to a very light violet in color. As larvae pass through successive instars they become progressively more violet in color. The final fifth instar (up to 25 mm in length) is characterized by having violet dorsal coloration contrasting strikingly with a blue-turquoise colored ventral surface. When fifth instar larvae are ready to pupate the majority (>95%) will abandon the seed or fruit within which they are feeding, initiate active walking and climbing for approximately 12 hours, after which they will enter a quiescent period for another ~12 hours, often in a protected spot (e.g., under paper towel in the lab). During this quiescent period larvae will spin a very lose and fragile silk tent within which they will pupate.
Pupae. Pupae are “free” chrysalises that may be loosely attached with fine weak silk strands to a substrate but can be easily dislodge with gentle prodding. In the field, S. catenifer pupate in the soil at 0.5-2.0cm depth after leaving the fruit they were feeding in (Boscán de Martínez and Godoy, 1984). Young pupae are a striking turquoise blue in color and within 4-8 hrs of initial pupation this color becomes reddish-brown as pupae mature and melonize. Male and female pupae can be separated based on the presence of a small “suture” that divides abdominal segment 9 in the males (Cervantes Peredo et al., 1999). Some S. catenifer larvae (<5%) will pupate within the seed in which they were feeding (Hoddle unpublished data).
Adults. Adult moths are light tan color, and wings are marked with numerous black spots. The most characteristic marking on the forewings is the easily observable “C” shape of spots as the distal end of the forewings. Adult females are about 15 mm in length (tip of head to tip of wings) when in the resting position with wings folded across the dorsum. Wing span for females with forewings fully spread is around 28-30 mm in breadth. Males tend to be slightly smaller (2-3 mm shorter) than females and are similarly colored (Hoddle unpublished data 2007).
During the day, the majority of adult S. catenifer rest in dried leaf duff, weeds, and other litter in avocado orchards and this behavior has been recorded in more natural forest situations as well (Cervantes et al., 1999). Flight monitoring of 45 laboratory-reared adult S. catenifer into an avocado orchard at 2:00pm in the afternoon revealed the following: (1) Adults (n=15) released individually at 1.5 meters on open ground in full sun all flew immediately into orchard weeds and dried avocado leaves and hid. (2) Adults (n=15) released individually at 1.5 meters immediately under the shade of an avocado canopy all flew directly to the ground and similarly hid amongst and under dried avocado leaves or in soil cracks. (3) Adults (n=15) released individually at ~3.5 meters of height within the canopy of avocado trees all flew directly to the ground to seek refuge. The distance measured for adult Stenoma flight upon release from plastic vials ranged from 3 to 12 meters.
Feeding Damage, Oviposition Preferences, Influence of Altitude on Distribution, and Avocado Cultivar Preferences
The major economic damage caused by S. catenifer larvae is feeding inside fruit and subsequent damage to seeds and fruit pulp. Infested fruit hanging in trees with obvious S. catenifer damage are characterized by whitish exudates (a mixture of seven carbon sugars composed primarily of perseitol) that run down the side of the fruit, accumulations of frass kick outs at the end of feeding galleries, and easily observable holes in the side of fruit. Opened fruit may show frass accumulation in the void that forms around the seed as the pulp separates from the seed as the fruit matures, seeds with feeding holes, or seeds completely destroyed by larval feeding can be found inside opened fruit. Complete seed destruction can occur in small fruit that harbor more than two S. catenifer larvae. Surveys of Hass avocados collected from commercial orchards in Guatemala have revealed that 1-2 larvae per seed is the most common level of infestation. However, it is not uncommon to find the occasional seed with 3-4 S. catenifer larvae, and occasionally 7-8 larvae may be found feeding in one seed. In Brazil >55% of avocado fruit that are attacked by S. catenifer are in the upper half of the tree (Hohmann et al., 2003). Surveys of damaged Hass fruit in Guatemala indicate that 8% of S. catenifer holes are in the top 1/3 of the fruit, 38% are in the middle third, and 54% are found in the bottom 1/3 of the fruit. Approximately 14% of fruit exhibit more than one S. catenifer hole. It is possible that fruit infested with S. catenifer larvae fruit may be prematurely aborted and will drop to the ground where larvae continue feeding in the seeds before emerging from fruit to pupate in the soil.
| Figure 8. Infested fruit hanging in trees with obvious S. catenifer damage are characterized by whitish exudates (a mixture of seven carbon sugars composed primarily of perseitol) that run down the side of the fruit, accumulations of frass kick outs at the end of feeding galleries, and easily observable holes in the side of fruit. | ||
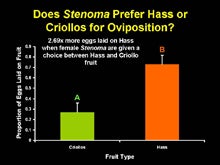
In the laboratory, when given a choice between Hass avocados or Criollos that are 85% mature, Stenoma females will lay significantly more eggs, up to 2.69 times more eggs on Hass fruit than Criollos. The reason for this preference is unknown, but it is possible that ovipositing female Stenoma can distinguish between fruit types of different qualities and preferentially lay more eggs on higher quality fruit. Hass is well known for its high oil content, a characteristic that makes this cultivar popular with humans. Stenoma may also prefer lipid rich avocados as hosts for offspring. Alternatively, the rough bumpy texture of Hass fruit in comparison to smoother skinned Criollos may be attractive to ovipositing females. Oviposition studies in the laboratory have demonstrated that Stenoma prefers to lay on substrates that have a rough texture (Nava et al., 2005a). Whatever the stimulus (oil content or rough skin), under laboratory conditions it appears that Hass avocados are a preferred variety for ovipositon by Stenoma females. Surveys of Hass avocados in Guatemala support this suggestion that Hass are a preferred cultivar as an average of 45% fruit in some commercial orchards exhibit feeding damage by Stenoma larvae.
|
Figure 9. Where do Stenoma larvae attack the fruit? Around 54% of attacks occur on the lower third of the fruit, with 145 of the fruit have more than one Stenoma hole. |
Figure 10. In Guatemala, S. catenifer infestations of avocados appear to be limited to altitudes below 5,200 feet. At higher altitudes (>5,500 feet) S. catenifer is very uncommon. |
Figure 11. Avocado varieties vary in their susceptibility to S. catenifer. Attack rates may be as low as an average of 4.5% of fruit in Booth, intermediate in Fuerte at 23%, and high in Rincon at 54% damage (Hohmann et al., 2000). |
Stenoma catenifer in the Galapagos Islands |
|
Figure 12. Avocados at the Farmer's Market in Puerto Ayora, Santa Cruz. Stenoma-infested fruit of South America can be quickly spread not only across the island of Santa Cruz but also between islands as people move fruit on boats. |
Figure 13. Stenoma damage to fresh avocados being sold in a supermarket in Puerto Ayoro, Santa Cruz in October 2009. The fruit in the foreground shows fresh Stenoma kick out from a live larva inside the fruit. |
Stenoma is not native to the Galapagos Islands and importation of avocados infested with seed moth larvae in 2001 from Ecuador on the mainland of South America resulted in the rapid establishment (i.e., within 12 months) of the seed moth on islands (i.e., Santa Cruz and San Cristobal) with avocado production. Seed moth is the only pest attacking avocados in the Galapagos, and infestation rates of fruit now approach 90% for the bi-annual crop. Infested fruit either drop prematurely from trees or are so badly damaged that they are unfit for consumption.
Consequently this highly prized fruit is no longer a viable crop, either for personal consumption or for sale in local produce markets. This problem has necessitated increased imports of fresh avocados from mainland South America which has increased food costs for local people, and further increases the risk of additional pests entering the Galapagos Islands.
|
Figure 14. Cotesia (Apanteles) spp. parasitoids emerging from S. cateniferlarva. |
Figure 15. Cotesia spp. cocoons on S. cateniferlarva. |
|
Figure 16. Cotesia spp. cocoons in an avocado seed. |
Figure 17. Gregarious Apanteles sp. |
|
Figure 18. The second most important parasitoid attacking S. catenifer in Guatemala is a solitary braconid, Macrocentrus sp. Adult parasitoids are dark yellow in color with brown markings. |
|
|
Figure 19. A single pale colored parasitoid larva emerges from hosts which have a contracted dehydrated appearance. |
Figure 20. Macroncentrus larvae spin a very distinctive beige colored and cigar shaped cocoon. |
In October 2009, 40 dropped fruit and exposed seeds were collected from under avocado trees around Bella Vista on the island of Santa Cruz. Of these 40 fruit, 21 Stenoma larvae were reared. Twenty larvae developed into pupae. One larva died from parasitism similar to that caused by Apanteles sp. in Guatemala (see below). However, all nine parasitoid larvae died as pupae, suggesting that parasitism may have been the opportunistic exploitation of an unsuitable host. Seven of the 20 pupae died and 13 adults emerged (62% larval to adult survivorship rate).
Natural enemies of Stenoma catenifer. In Brazil, S. catenifer eggs are attacked by Trichogramma sp. and Trichogrammatoidea sp. parasitoids and up to ~60% of eggs can be parasitized. However, this level of attack is not high enough to prevent economic damage (Hohmann et al., 2003). Larvae in Brazil are attacked by a variety of hymenopterous parasitoids which can cause up to 30-40% parasitism. Larval parasitoids recorded from S. catenifer larvae include: Cotesia (Apanteles) spp. Dolichogenidea sp., Hypomicrogaster sp., Chelonus sp., and Hymenochaonia sp. (all Hymenoptera: Braconidae) (Nava et al., 2005b). Ichneumonids have also been recorded from S. catenifer larvae and include: Eudeleboea sp., and Pristomerus sp. (Nava et al., 2005b).
The second most important parasitoid attacking S. catenifer in Guatemala is a solitary braconid, Macrocentrus sp. Adult parasitoids are dark yellow in color with brown markings. This parasitoid causes third and fourth instar S. catenifer larvae to abandon avocado fruit before fully maturing. A single pale colored parasitoid larva emerges from hosts which have a contracted dehydrated appearance. Macroncentrus larvae spin a very distinctive beige colored and cigar shaped cocoon. In Guatemala, the dominant larval parasitoid attacking S. catenifer is a gregarious Apanteles sp. which can succcessfully parasitize 50-100% of larvae. Estimates of parasitism levels from surveys in Guatemala are affected by location, number and time samples were taken (Hoddle unpublished data 2007). Young Apanteles sp. larvae erupt out fifth instar S. catenifer larvae and about 4-8 hours after emergence the majority of larvae have spun silk cocoons. The number of parasitoid larvae emerging from S. catenifer larvae ranges 2-27, and averages 8-9 larvae per host. A small number of S. catenifer larvae die in avocado seeds and parasitoids pupate within larval feeding galleries within the avocado seed. Adult parasitoids are black with orange coxae. The sex ratio is around 53% male and 87% of adult parasitoids successfully emerge from cocoons.
Terrestrial predators are likely to be important natural enemies of S. catenifer because mature S. catenifer larvae emerge from the protection of avocado seeds to pupate in the soil and adult moths seek refuge in leaf duff on the orchard floor during day light hours. One very common ground predator encountered in Guatemalan avocado orchards during the day and night was the lycosid spider Hogna sp. In laboratory trials, this predator readily consumed larval, pupal, and adult S. catenifer. Spiders were still alive 36 hrs post-feeding indicating that immature and adult S. catenifer life stages did not have any adverse intoxicating effect on these predators.
Monitoring and Rearing Stenoma catenifer |
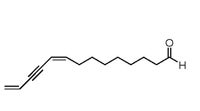
| Figure 21. A structural diagram of the S. catenifer sex pheromone, (9Z)-9,13-tetradecadien-11-ynal, a new class of natural compound. |
| Figure 22. A wing trap freshly baited with a gray rubber septum loaded with the sex pheromone. |
| Figure 23. A trap with male S. catenifer that were captured in a commercial Hass avocado orchard in Guatemala after a four night deployment period. |
| Figure 24. An adult Antaeotricha nictitans (note the two spots in the center of the forewings). |
| Figure 25. An adult Stenoma catenifer (note the multiple spots on the forewings. |
The sex pheromone of Stenoma catenifer. The sex pheromone of S. catenifer has been identified as (9Z)-9,13-tetradecadien-11-ynal (Millar et al. 2008), and field tests have demonstrated that this single component is attractive to male moths (Hoddle et al. 2009). The best lure for dispensing the pheromone is a 11 x 5 mm gray rubber septum (West Pharmaceutical Services Inc., Lionville PA, Product #10600275) that is placed in a Trece wing trap and hung at a height of 1.75 m inside the canopy of an avocado tree. The pheromone is attractive to male moths for at least four weeks. Empirical testing to determine the number of traps to be deployed on a per orchard basis suggests that just 10-13 traps randomly deployed throughout commercial orchards for seven days when there is no obvious S. catenifer damage will catch at least one male moth with about 90% confidence. Flight distance studies using pheromone traps and male moths dusted with DayGlo dust have indicated that in a single night, male moths may fly around 67 m. However, this measurement may be an underestimate (Hoddle et al., 2009a).
In low elevation orchards (˜400-700 m) in Mexico and Guatemala, a second Stenomatinae moth, Antaeotricha nictitans, was captured 7-9 times more frequently than S. catenifer in wing traps baited with the S. catenifer sex pheromone. The association of A. nictitans with avocado orchards is suggestive that this moth may be an unknown pest of avocados at low elevations. A. nictitans is easy to identify when compared to S. catenifer because it is a larger moth, and the pattern of spots on the forewings is different. When the forewings are compared, A. nictitans has only a single pair of spots on the forewings, that is a single spot in the middle of each forewing. In comparison, S. catenifer has many more spots, especially at the distal margin of the forewing where the dots form a "C" shape, similar to a clown's painted mouth.
Traps baited with synthetic sex pheromone have consistently captured S. catenifer in three different countries (Guatemala, Mexico, and Brazil) suggesting that the existence of different pheromone races of S. catenifer is unlikely.
The information provided above is sufficient to develop effective protocols for using the S. catenifer pheromone to detect and monitor this pest in countries with endemic populations in commercial orchards that are exporting fresh avocados, and for quarantine detection and incursion monitoring in countries receiving avocado imports from high risk areas.
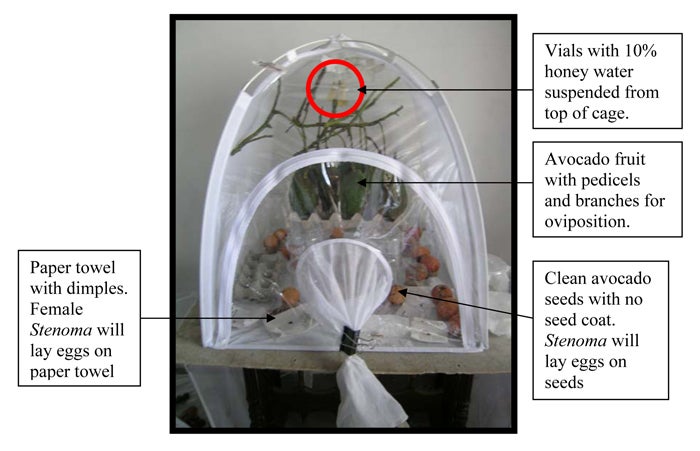
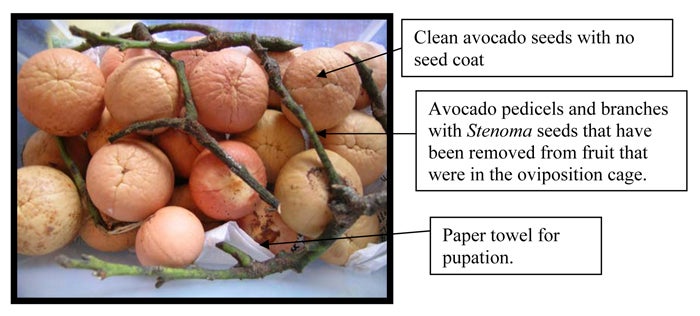
Establishing and maintaining a Stenoma catenifer colony. Introduce adult moths into a cage (e.g., commercially available bugdorms [see photo] are perfect rearing cages for adult S. catenifer) and supply adults with 10% honeywater in a vial with cotton wick suspended from the roof of the cage. Honeywater is a food source and should be changed every 5 days as it begins to ferment. Provide clean avocado fruit to stimulate oviposition. Fruit should be thoroughly washed and dried to remove contaminants (e.g., dirt, predatory mites, scales, and mealy bugs) before introducing into the cage. Female Stenoma will oviposit on fruit, pedicels, and branches attached to fruit. Female moths will also lay eggs on dimpled paper towels (Nava et al., 2005a) and clean avocado seeds that are placed in the cage.
Every seven days remove fruit, seeds, and paper towel from cage. Replace with new fruit, seeds, and paper towel. With the removed fruit, seeds, and paper towel do the following things:
1) Cut pedicel (leave about 1-1.5 cm of pedicel on the fruit. This will reduce the rate at which fungus will grow into the fruit from the exposed button) from fruit and place in a box with clean fresh avocado seeds that have had the seed coat (testa) removed. Seed coat removal is very easy. Crack open ripe, uninfested avocados that may be just starting to show signs of mold/fungus growth. Remove the seed and clean vigorously with paper towel. The coarse paper towel will remove the seed coat. Seed coat removal has two advantages: (i) it reduces greatly the likelihood of fungus growing on the seed, and (ii) very young Stenoma that hatch from eggs on the pedicels and branches can more easily burrow into the clean seeds. Place clean avocado seeds and avocado pedicels/branches that have Stenoma eggs in a ventilated sealed container with two layers of paper towel. The eggs will hatch on the avocado pedicel/branch and the young larvae will attack the seeds. At this time remove paper towels and seeds from the oviposition cage as these may have eggs and place in a ventilated container with clean avocado seeds and with two layers of paper towel (larvae will pupate between the layers of paper towel). Larvae hatching from eggs on the paper towel will burrow into the clean avocado seeds and commence feeding.
2) Remove the avocado fruit with eggs from the oviposition cage and place in either a sealed, ventilated container or another cage. Leave the fruit undisturbed for about 2 - 2.5 weeks. During this time the Stenoma eggs will hatch and the young larvae will burrow through the avocado pulp to the seed to start feeding. After 2 - 2.5 weeks crack open the fruit, extract the seed, clean and place in a ventilated container with two layers of paper towel. The larvae will continue to feed and develop within the cleaned seeds.
3) Every week get 40-50 avocado fruit to use for egg laying in the oviposition cage (about 12 fruit in the cage). The unused fruit should be opened when ripe and the seeds extracted and cleaned for feeding to young caterpillars.
4) After 3-4 weeks the mature Stenoma caterpillars will exit the seeds and begin vigorous walking in the container for about 12 hrs. After this time the majority will hide between the sheets of paper towel and will begin to pupate. After an additional 12-24 hrs remove pupae from ventilated container and place in the oviposition cage or seperate for use in experiments.
5) After about 10 days the pupae will finish development and the adult moths will emerge into the oviposition cage. The adults will mate and lay eggs in the cage and the cycle will repeat.
References |
- Acevedo, E., Vasquez, J., and Sosa, C. 1972. Estudios sobre el barrendor del heuso del aguacate Stenoma catenifer Walsingham (Lepidoptera: Stenomidae). Agrociencia 9: 17-24.
- Boscán de Martínez, N. and Godoy, F.J. 1982. Apanteles sp. (Hymenoptera: Braconidae) parasito del taladrador de fruto del aguacate Stenoma catenifer Walsingham (Leipidoptera: Stenomidae) en Venezuela. Agronomia Tropical 32: 319-321.
- Boscán de Martínez, N. and Godoy, F.J. 1984. Observaciones preliminares sobre la biologia de Stenoma catenifer Walsingham (Lepidoptera: Stenomidae) taladrador del aguacate (Persea americana Mill.). Agronomia Tropical 34: 205-208.
- Cervantes Peredo, L., Lyall, C.H.C., and Brown, V.K. 1999. The stenomatine moth, Stenoma catenifer Walsingham: a pre-dispersal seed predator of Greenheart (Chlorocardium rodiei [Schomb.] Rohwer, Richter and van der Werff) in Guyana. Journal of Natural History 33: 531-542.
- Garcia, A.M., Villa, M.M., Gutiérrez, A.M. 1967. El aguacate: plagas and enfermades. Fitofilo 20: 5-30.
- Hoddle, M.S., and C.D. Hoddle. 2008a. Bioecology of Stenoma catenifer (Lepidoptera: Elachistidae) and associated larval parasitoids reared from Hass avocados in Guatemala. J. Econ. Entomol. 101: 692-698.
- Hoddle, M.S., and C.D. Hoddle. 2008b. Lepidoptera and associated parasitoids attacking Hass and non-Hass avocados in Guatemala. J. Econ. Entomol. 101: 1310-1316.
- Hoddle, M.S., and C.D. Hoddle. 2008c. Aspects of the field ecology of Stenoma catenifer (Lepidoptera: Elachistidae) infesting Hass avocados in Guatemala. Fla. Entomol. 91: 693-694.
- Hoddle, M.S., J.G. Millar, C.D. Hoddle, Y. Zou, and J.S. McElfresh. 2009. Synthesis and field evaluation of the sex pheromone of Stenoma catenifer (Lepidoptera: Elachistidae). J. Econ. Entomol. (in press).
- Hoddle, M.S., J.G. Millar, C.D. Hoddle, Y. Zou, J.S. McElfresh, and S.M. Lesch. 2009. Field optimization of the sex pheromone of Stenoma catenifer (Lepidoptera: Elachistidae): Evaluation of lure types, trap heights, male flight distances, and number of traps needed per orchard for detection. Journal of Economic Entomology (submitted).
- Hohmann, C.L., Dos Santos , W.J., Menegium, A.M. 2000. Avaliação de técnicas de manejo para o controle de broca-do-abacate, Stenoma catenifer (Wals) (Lepidoptera: Oecophoridae). Rev. Bras. Frutic. Jaboticabal 22: 359-363.
- Hohmann, C.L., Meneguim, A.M., Andrade, E.A., Novaes, T.C., and Zandoná C. 2003. The avocado fruit borer Stenoma catenifer (Wals.) (Lepidoptera: Elachistidae): egg and damage distribution and parasitism. Revista Brasileria de Fruticultura 25: 432-435.
- Millar, J.G., M.S. Hoddle, J.S. McElfresh, Y. Zou, and C.D. Hoddle. 2008. (9Z)-9,13-tetradecadien-11-ynal, the sex pheromone of the avocado seed moth, Stenoma catenifer. Tetrahedron Letters 49: 4820-4823.
- Nava D.E., Parra, J.R.P., Diez-Rodríguez, Bento. J.M.S. 2005a.Oviposition behavior of Stenoma catenifer (Lepidoptera: Elachistidae): chemical and physical stimuli and diel pattern of egg laying. Annals of the Entomological Society of America 98: 409-414.
- Nava D.E., Parra, J.R.P., Costa, V.A., Guerra, T.M., and Cônsoli, F.L. (2005b). Population dynamics of Stenoma catenifer (Lepidoptera : Elachistidae) and related larval parasitoids in Minas Gerais, Brazil. Florida Entomologist 88: 441-446.
- Nava D.E., de Lara Haddad, M., and Parra, J.R.P. 2005c. Exigências térmicas, estimativa do número de geroções de Stenoma catenifer e comprovação de modelo em campo. Pesq. Agropec. Bras., Brasilia 40 : 961-967.
- Ventura, M.U., Destro, D., Lopes, E.C.A., and Montalván, R. 1999. Avocado moth (Lepidoptera: Stenomidae) damage in two avocado cultivars. Florida Entomologist 82: 625-631.